Carnitine is a compound in the body that helps your body digest fats for energy. A carnitine deficiency is related to a number of different medical problems. A carnitine total and free plasma test is a blood test that measures the amount of carnitine in the blood. It examines that amount of usable, or free, carnitine and compares it with the total amount of carnitine.
The analysis of carnitines is indicated in people who exhibit the following:
- failure to thrive,
- hypotonia,
- chronic muscle weakness,
- cardiomyopathy,
- intermittent episodes of weakness and encephalopathy,
- renal Fanconi's syndrome,
- hypoglycemic episodes,
- metabolic acidosis,
- or hypoketotic dicarboxylic acidurias.
------------------
Reference Ranges:
Carnitine is a quaternary, water-soluble ammonia compound biosynthesized from lysine and arginine. It serves as a mechanism for transport of long-chain fatty acids from the cytoplasm across the inner mitochondrial membrane and into the mitochondrial matrix, the site of b-oxidation of fatty acids for energy generation.
The reference range of carnitine depends on the laboratory being used;
Reference Range of Carnitine at a Single Laboratory
|
Age Range |
Serum Free Carnitine (µmol/L) |
Serum Total Carnitine (µmol/L) |
|
Neonate |
26-76 |
35-102 |
|
Child |
41.4±10.4* |
56.2±11.4* |
|
Adolescent female |
39.3±8.1* |
53.2±8.9* |
|
Adolescent male |
39.6±9.3* |
53.5±10.5* |
|
Adult female |
19.3-53.9** |
28.1-66.4** |
|
Adult male |
38.8-69.5 |
44.2-79.3 |
|
*Mean ± standard deviation (SD) **95% confidence interval (CI) |
||
Quest Laboratories reports the reference range of total carnitine as follows:
-
Men: 30-70 μmol/L
-
Women: 25-58 μmol/L
-
Male children (age ≤17 years): 32-62 μmol/L
-
Female children (age ≤17 years): 28-59 μmol/L
Quest Laboratories reports the reference range of free carnitine as follows:
-
Men: 23-59 μmol/L
-
Women: 19-48 μmol/L
-
Male children (age ≤17 years): 25-54 μmol/L
-
Female children (age ≤17 years): 19-51 μmol/L
The University of California San Francisco Laboratory reports the normal values of free and total carnitine in adults as 18-69 μmol/L and 20-71 μmol/L, respectively.
Chace et al (2003) examined free and total carnitine levels in newborns. The reference ranges depended both on technique used (radioenzyme vs tandem mass spectroscopy) and the sample type (whole blood vs serum).
Variations in the ratio of free to total carnitine may also be important; typically, normal is reported as 0.1-0.4.
References:
Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Validated Method for the Quantification of Free and Total Carnitine, Butyrobetaine, and Acylcarnitines in Biological Samples. Anal Chem. 2015 Sep 1. 87 (17):8994-9001. [QxMD MEDLINE Link].
Belay B, Esteban-Cruciani N, Walsh CA, Kaskel FJ. The use of levo-carnitine in children with renal disease: a review and a call for future studies. Pediatr Nephrol. 2006 Mar. 21(3):308-17. [QxMD MEDLINE Link].
Quest Diagnostics. Available at http://www.questdiagnostics.com/testcenter/%20BUOrderInfo.action?tc=5800&labCode=AMD.
Chace DH, Pons R, Chiriboga CA, et al. Neonatal blood carnitine concentrations: normative data by electrospray tandem mass spectometry. Pediatr Res. 2003 May. 53(5):823-9. [QxMD MEDLINE Link].
Stanley CA. Carnitine deficiency disorders in children. Ann N Y Acad Sci. 2004 Nov. 1033:42-51. [QxMD MEDLINE Link].
Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012 Sep 1. 51(9):553-72. [QxMD MEDLINE Link].
Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006 May 15. 142C(2):77-85. [QxMD MEDLINE Link]. [Full Text].
Dahash BA, Sankararaman S. Carnitine Deficiency. StatPearls. 2022 Jan. [QxMD MEDLINE Link]. [Full Text].
Santra S, Hendriksz C. How to use acylcarnitine profiles to help diagnose inborn errors of metabolism. Arch Dis Child Educ Pract Ed. 2010 Oct. 95(5):151-6. [QxMD MEDLINE Link].
Scaglia F. Carnitine Deficiency. Medscape Drugs & Diseases. Updated 2019 Dec 13. [Full Text].
Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012 Sep 18. 7:68. [QxMD MEDLINE Link]. [Full Text].
Tein I. Metabolic myopathies. Swaiman KF, Ashwal S, Ferriero DM. Swaiman’s Pediatric Neurology Principles and Practice. 5th ed. Schor NF: Elsevier Sanders; 2012. 1627-40.
Crefcoeur LL, Visser G, Ferdinandusse S, Wijburg FA, Langeveld M, Sjouke B. Clinical characteristics of primary carnitine deficiency: A structured review using a case-by-case approach. J Inherit Metab Dis. 2022 May. 45 (3):386-405. [QxMD MEDLINE Link]. [Full Text].
Berardo A, DiMauro S, Hirano M. A diagnostic algorithm for metabolic myopathies. Curr Neurol Neurosci Rep. 2010 Mar. 10(2):118-26. [QxMD MEDLINE Link]. [Full Text].
Bernardini I, Rizzo WB, Dalakas M, Bernar J, Gahl WA. Plasma and muscle free carnitine deficiency due to renal Fanconi syndrome. J Clin Invest. 1985 Apr. 75(4):1124-30. [QxMD MEDLINE Link]. [Full Text].
Gahl WA, Bernardini I, Dalakas M, et al. Oral carnitine therapy in children with cystinosis and renal Fanconi syndrome. J Clin Invest. 1988 Feb. 81(2):549-60. [QxMD MEDLINE Link]. [Full Text].
Determination of Free and Total Carnitine and Choline in Infant Formulas and Adult Nutritional Products by UHPLC-MS/MS: Single-Laboratory Validation, First Action 2014.04. J AOAC Int. 2015 Jun 24. [QxMD MEDLINE Link].
El-Hattab AW. Systemic Primary Carnitine Deficiency. 1993. [QxMD MEDLINE Link].
Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Genet Med. 2008 Feb. 10(2):151-6. [QxMD MEDLINE Link].
Dietzen DJ, Rinaldo P, Whitley RJ, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry; executive summary. Clin Chem. 2009 Sep. 55(9):1615-26. [QxMD MEDLINE Link].
Johns Hopkins University Clinical Lab.
National Newborn Screening Status Report 2012.
Stanley CA. Carnitine disorders. Adv Pediatr. 1995. 42:209-42. [QxMD MEDLINE Link].
Mamedov I, Zolkina I, Nikolaeva E, Glagovsky P, Sukhorukov V. Carnitine insufficiency in children with inborn errors of metabolism: prevalence and treatment efficacy. J Pediatr Endocrinol Metab. 2015 Jul 18. [QxMD MEDLINE Link].
Sgambat K, Moudgil A. Carnitine deficiency in children receiving continuous renal replacement therapy. Hemodial Int. 2015 Aug 11. [QxMD MEDLINE Link].
What does it mean if your Carnitine, Free result is too high?
Carnitine, specifically in its free form, is a biologically essential quaternary ammonium compound that plays a pivotal role in the metabolism of fatty acids within the mitochondria of cells. Free carnitine acts as a critical transporter, facilitating the movement of long-chain fatty acids from the cytosol into the mitochondrial matrix, where these fatty acids undergo β-oxidation to generate adenosine triphosphate (ATP), the cell's primary energy currency.
This process is vital for energy production, especially in metabolically active tissues such as the heart, skeletal muscles, and liver. The body synthesizes carnitine from the amino acids lysine and methionine, with its synthesis and regulation involving several enzymes and vitamin C as a cofactor, indicating a complex biochemical pathway that underscores its importance in cellular metabolism.
Elevated levels of free carnitine in the body could indicate a variety of conditions but primarily suggest an increased availability or intake of carnitine, either through diet or supplementation. It may also reflect metabolic differences or inefficiencies in how the body uses carnitine, such as in certain liver disorders where carnitine is not utilized properly, leading to its accumulation in the blood. In some cases, high carnitine levels can be a marker of improved carnitine synthesis or decreased usage by the body's tissues, especially if there are underlying metabolic conditions affecting energy production. However, elevated carnitine levels are relatively uncommon and usually not a cause for concern unless accompanied by other symptoms or diagnosed conditions, making it important to consult healthcare professionals for accurate diagnosis and interpretation.
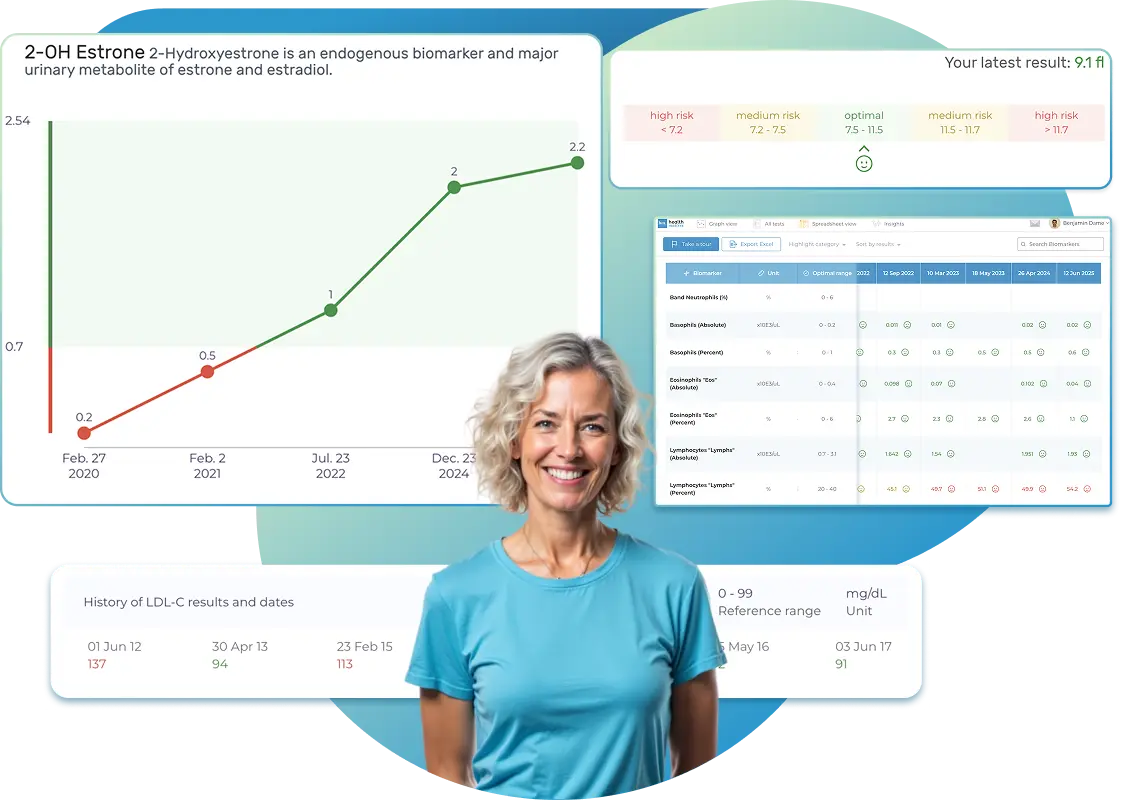
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your Carnitine, Free result is too low?
Primary carnitine deficiency is caused by an autosomal-recessive defect in the SLC22A5 gene, resulting in a lack of OCTN2, which is a high-affinity carnitine-uptake transporter expressed in muscle, kidney, and heart.
Laboratory values in primary carnitine deficiency show markedly decreased free and total carnitine levels, since 90-95% of filtered carnitine is lost in the urine. Analysis of urine organic acids, serum amino acids, and acylcarnitine panels can be used to distinguish this condition from other causes of carnitine deficiency.
Individuals with primary carnitine deficiency usually present with cardiomyopathy and skeletal weakness or with episodic hypoketotic hypoglycemia and encephalopathy when stressed at around age 2-4 years. This results from the inability to oxidize fatty acids and generate ketones to provide energy during catabolic states. The disorder is fatal without treatment, but supplementation with oral carnitine results in elevated carnitine levels and prevents progression of the disease.
A literature review by Crefcoeur et al found that in individuals with primary carnitine deficiency, the most prevalent symptoms were cardiac (23.8% of patients), with cardiomyopathy being the predominant manifestation of these. Neurologic, hepatic, and metabolic symptoms developed in 7.1%, 8.4%, and 9.2% of persons with primary deficiency and occurred most often in early childhood. The condition was asymptomatic in 55.1% of patients with primary deficiency.
Carnitine-acylcarnitine translocase deficiency (CACT) typically presents in an autosomal-recessive fashion with seizures, apnea, and an irregular heart beat in the neonatal period (although presentation can occur as late as age 15 months) and results from mutations in the CACT protein (SLC25A20 gene), a carnitine-acylcarnitine exchanger on the inner mitochondrial membrane. Crisis is triggered by fasting, viral illness, or stress (an in other fatty-acid disorders). In addition to low carnitine levels, laboratory studies also show hypoketotic hypoglycemia; elevated levels of ammonia, creatine kinase (CK), liver enzymes, and long-chain acylcarnitines in the blood; and dicarboxylic aciduria in urinary organic acids. CACT is treated with frequent feedings of carbohydrates, medium-chain triglycerides, and carnitine.
The autosomal-recessive disorder carnitine palmitoyltransferase 2 (CPT-2) deficiency is also characterized by low carnitine levels. The CPT-2 protein is essential for removing carnitine from long-chain fatty acids after translocation into the mitochondrial matrix is and thus essential for fatty acid oxidation. Although it typically presents as a myopathy in adolescents or adults, CPT-2 deficiency can also present as severe fatal neonatal and hepatocardiomuscular infantile forms. The difference in presentation relates to the amount of residual function (genotype-phenotype correlation).
Neonates with CPT-2 deficiency present within days of birth with encephalopathy, cardiomegaly, hepatomegaly, seizures, cardiac arrhythmias, and respiratory distress, and the condition is rapidly fatal. The infantile form presents between ages 6 and 24 months as episodes of encephalopathy, liver failure, seizures, hypoketotic hypoglycemia, metabolic acidosis, elevated CK levels, reversible hepatomegaly, and, in some cases, cardiomyopathy and arrhythmias, precipitated by infection, fasting, or fever.
The adolescent and adult form of CPT-2 deficiency presents with myopathic pain precipitated by exercise, cold, fever, or prolonged fasting and may be associated with myoglobinuria and kidney damage/failure.
Elevated long-chain acylcarnitine levels are detected in all forms of CPT-2 deficiency, and neonatal screening can be useful in determining the cause of death in the neonatal form.
Secondary carnitine deficiency can result from numerous conditions, such as chronic renal failure, end-stage renal disease, renal Fanconi syndrome, Lowe syndrome, cystinosis, and valproate therapy, all of which cause impaired carnitine reuptake from the kidneys. Carnitine-free diets (such as in those receiving intravenous nutrition), organic acidurias, and urea-cycle defects can also cause deficiency.
Transient falsely low carnitine levels have been reported in infants born to mothers with primary carnitine deficiency.
Laboratories
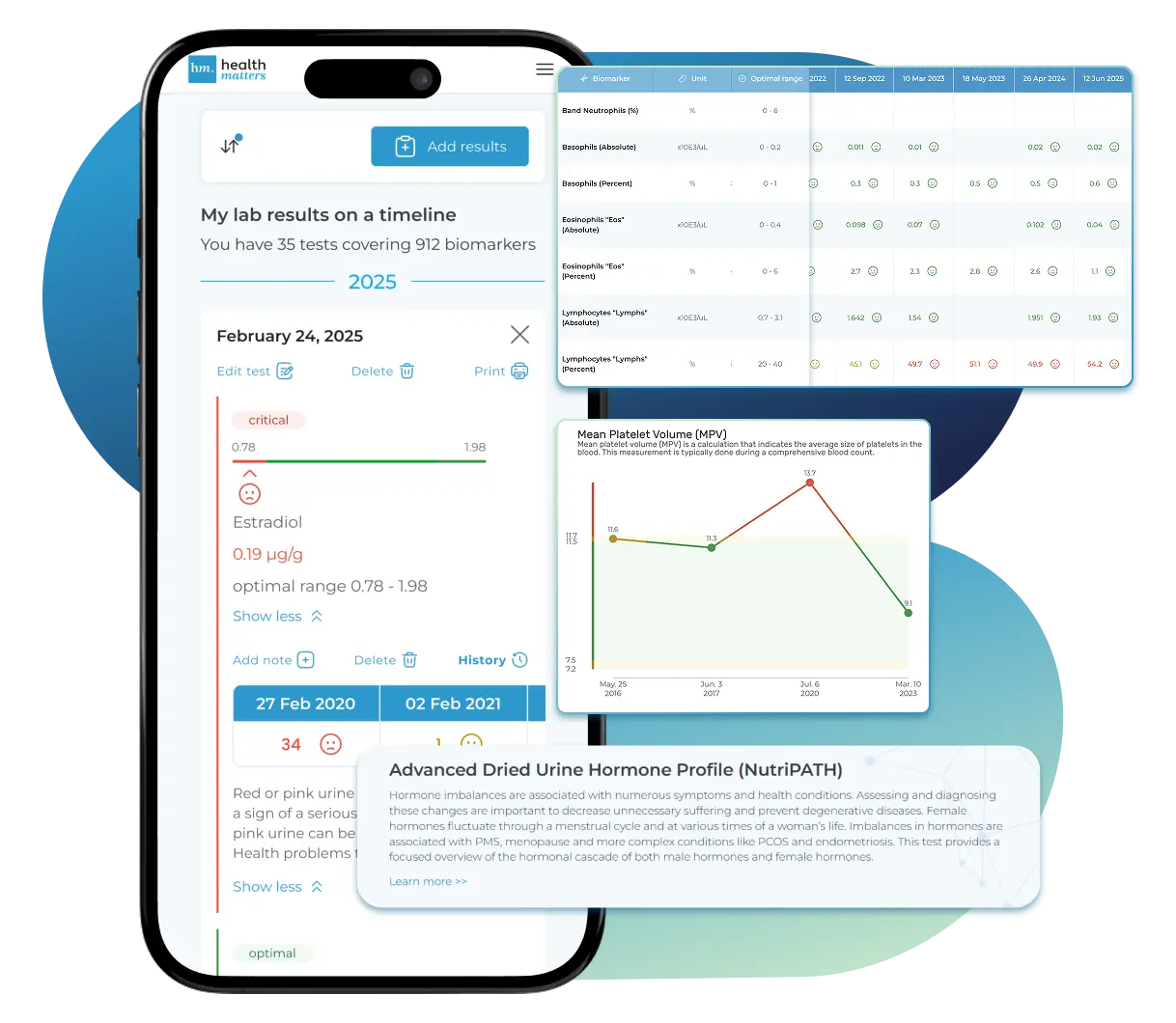
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















