b-Hydroxybutyric Acid
b-hydroxybutyrate is one of the ketone bodies.
The term ketone body describes any of 3 molecules: acetoacetate, b-hydroxybutyrate, or acetone. Acetoacetate is produced by acetyl-CoA metabolism, b-hydroxybutyrate is the result of acetoacetate reduction, and acetone is produced by the spontaneous decarboxylation of acetoacetate.
Ketone bodies are fundamental for metabolic homeostasis during periods of prolonged starvation. The brain cannot use fatty acids for energy production and usually depends on glucose to meet its metabolic needs. In cases of fasting or starvation, ketone bodies become a major fuel for brain cells, sparing amino acids from being catabolized to gluconeogenesis precursors to be used to supply the brain with energy. After prolonged starvation, ketone bodies can provide as much as two thirds of the brain's energy needs.
Ketone bodies are strong organic acids that fully dissociate in blood. When ketone body production becomes uncontrollable, the buffering systems are saturated, and blood pH drops; this is a condition known as ketoacidosis.
The two common clinical scenarios for ketoacidosis are diabetic ketoacidosis and alcoholic ketoacidosis.
Diabetic ketoacidosis
The most clinically relevant application of b-hydroxybutyrate determination involves the diagnosis, management, and monitoring of diabetic ketoacidosis. During states of insulin deficiency, lipolysis at the adipose tissue (stimulated by insulin deficiency) provides a huge fatty acid load to the liver. Fatty acids are initially metabolized to acetyl-coenzyme A that cannot enter the citric acid cycle in the mitochondria due to oxaloacetate deficiency. Thus, acetyl-coenzyme A is diverted to ketone body production through the activity of several enzymes, producing acetoacetate. Acetoacetate is then reduced to 3-b-hydroxybutyrate by 3-b-hydroxybutyrate dehydrogenase.
The ratio of acetoacetate to 3-b-hydroxybutyrate depends on the redox status in the liver mitochondria (ie, the NAD+/NADH ratio). Under normal circumstances, the b-hydroxybutyrate to acetoacetate ratio is around 1; however, in diabetic ketoacidosis, this may increase to 7-10. Acetone is produced by the spontaneous decarboxylation of acetoacetate.
Traditionally, the diagnosis of diabetic ketoacidosis was based on the detection of ketones in urine using the Legal reaction, during which acetoacetate reacts in the presence of alkali with nitroprusside to produce a purple-colored complex on a test strip. However, this method has significant drawbacks. It is semiquantitative and not equally sensitive for urine and blood.
Moreover, not all the patients with diabetic ketoacidosis are able to provide a urine sample upon presentation, and ketones in urine are not a precise estimation of blood ketones. Most importantly, the most abundant ketone body during diabetic ketoacidosis is b-hydroxybutyrate, with a concentration 3-10 times higher of that of acetoacetate. As diabetic ketoacidosis is treated, serum b-hydroxybutyrate is transformed to acetoacetate due to the correction of the mitochondrial redox status, elevating urine acetoacetate levels and giving the false impression that the patient has not responded to treatment.
Lastly, urine ketone strips can give false positive results in patients receiving drugs with sulfhydryl groups and false-negative results when they have been exposed to air for a long period of time or when the urine is acidic. These disadvantages necessitate the evolution of a more reliable method for the diagnosis and management of diabetic ketoacidosis.
Alcoholic ketoacidosis
This is the second most common cause of ketoacidosis, although significantly less common than diabetic ketoacidosis. In most cases, patients report significant alcohol consumption accompanied by fasting. From a biochemical point of view, ethanol is metabolized to acetoacetate and then acetate, producing significant amounts of NADH. In order to regenerate NAD+, pyruvate is metabolized to lactate and oxaloacetate is consumed to produce malate, depleting gluconeogenesis precursors. During starvation, insulin levels are extremely low and facilitate acyl-CoA entry into mitochondria, producing significant amounts of acetyl-CoA that cannot be metabolized in the Krebs cycle and is diverted towards ketone body synthesis.
In alcoholic ketoacidosis, the b-hydroxybutyrate to acetoacetate ratio is extremely high and b-hydroxybutyrate levels may be useful in the diagnosis and management of alcoholic ketoacidosis. However, no studies have been performed to actually compare b-hydroxybutyrate (serum or blood) with the traditional diagnostic parameters for alcoholic ketoacidosis and thus no recommendations can be made in favor or against its use in this setting.
What does it mean if your b-Hydroxybutyric Acid result is too high?
Elevated serum b-hydroxybutyrate levels can be observed in various conditions associated with metabolic substrate use disorders, insulin deficiency, and altered redox status, including the following:
→ Diabetic ketoacidosis: Ketone body production is stimulated by dehydration and insulin deficiency. Levels are usually more than 3 mmol/L.
→ Alcoholic ketoacidosis: Ketone body production is stimulated by altered redox status within the liver mitochondria.
→ High fat diet
→ Steroid or growth hormone deficiency
→ Salicylate poisoning
→ Fasting and starvation: Serum b-hydroxybutyrate levels are increased after approximately 3 days, rising to a plateau after 4 weeks of food deprivation.
→ Lactation: Ketone body production is stimulated by the high-fat content of milk.
→ Ketogenic diets: These diets are popular for the control of refractory seizures and body weight in obese individuals.
→ Glycogen-storage diseases and other metabolic disorders
A prospective study by Flores-Guerrero et al indicated that high plasma levels of b-hydroxybutyrate signal an increased risk of heart failure with reduced ejection fraction (HFrEF), especially in females. In terms of incident HF (which in these results was primarily due to HFrEF), the hazard ratio per one standard deviation increase in the b-hydroxybutyrate concentration was 1.40. More specifically, in women, one standard deviation was associated with a hazard ratio for HFrEF of 1.73, compared with 1.14 in men.
References:
Flores-Guerrero JL, Westenbrink BD, Connelly MA, Otvos JD, Groothof D, Shalaurova I, Garcia E, Navis G, de Boer RA, Bakker SJL, Dullaart RPF. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur J Clin Invest. 2021 May;51(5):e13468. doi: 10.1111/eci.13468. Epub 2020 Dec 18. PMID: 33616911; PMCID: PMC8244065.
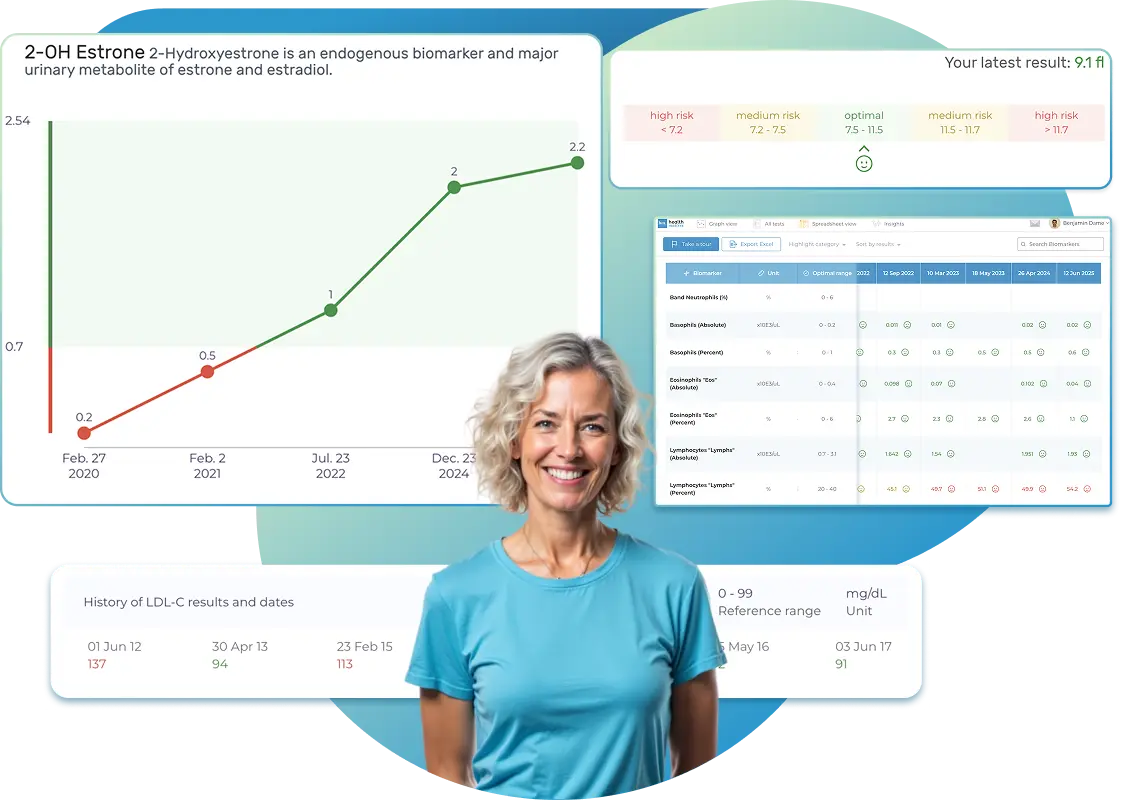
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your b-Hydroxybutyric Acid result is too low?
β-hydroxybutyric acid is a ketone and a byproduct of fatty acid metabolism and makes up ~70% of ketones produced in liver mitochondria.
Low levels of 3-Hydroxybutyric acid (=beta-hydroxybutyrate) may occur if there are low levels of precursors (fat, amino acids), if there are nutritional enzyme inhibitions, or if a low-activity enzyme variant is inherited. Ketone bodies are derived from fatty acids, levels may be lower on high carbohydrate diets. Ketogenesis occurs within the liver mitochondria, which are sensitive to oxidative stress and environmental toxins. Liver disorders may impair ketone synthesis and abnormally low levels of ketone bodies may impair liver functions.
Low levels of malate or orotate may also impair liver function.
→ Consider supporting synthesis pathways with vitamin B3 and phosphatidyl choline. Increasing healthy dietary fats, digestion and absorption may improve beta-hydroxybutyrate levels.
→ Review Citric Acid Cycle Metabolites and consider nutritional support for mitochondria.
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
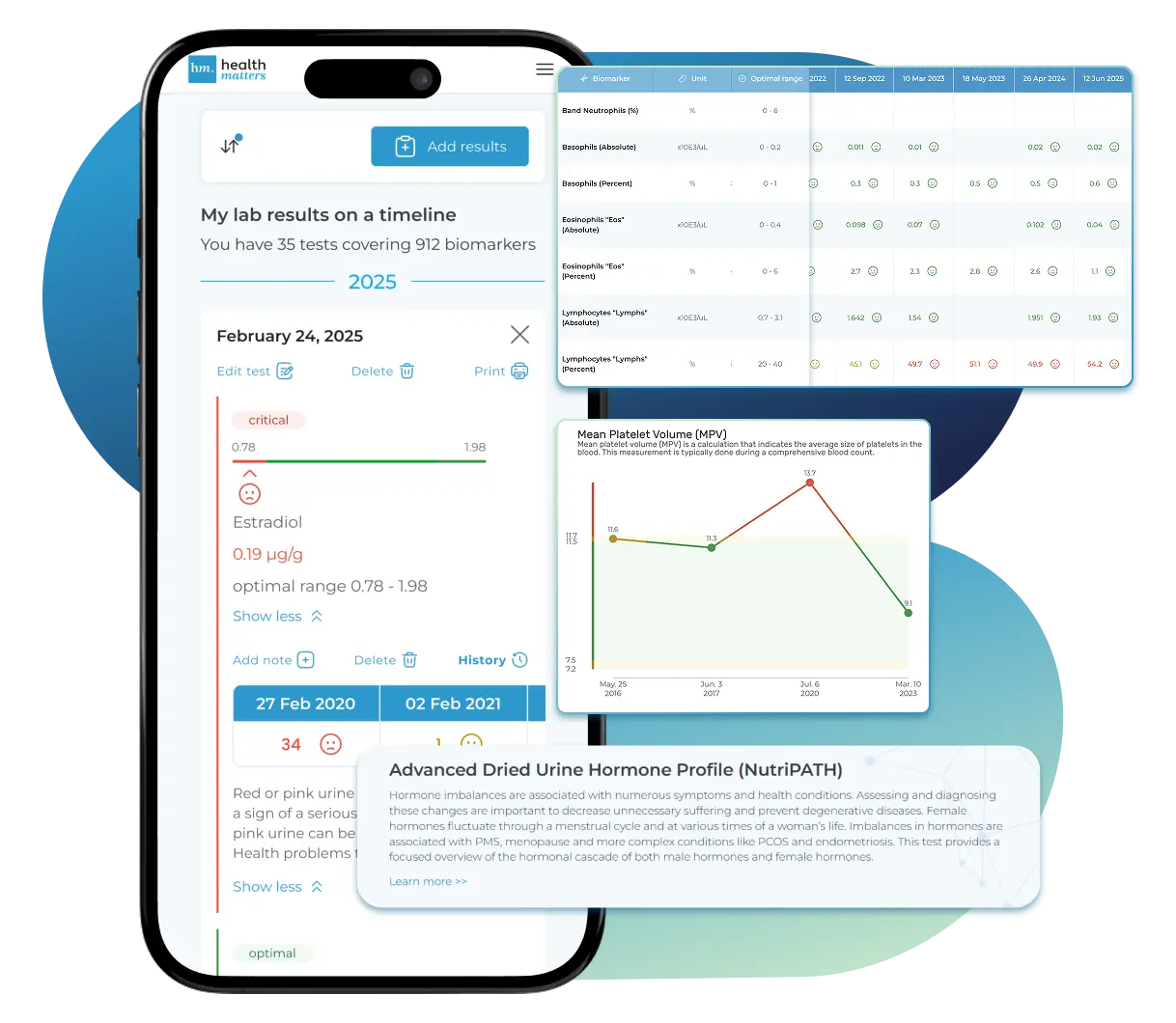
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















