Ruminococcus bromii
Ruminococcus bromii is a keystone species, playing a large role in the digestion of resistant starches. It has been proposed that the primary role played by R. bromii is to release energy from resistant starch to other members of the microbial community, giving it an important role for maintaining microbial community balance. R. gnavus can efficiently cross-feed on starch degradation products released by R. bromii, even though it is normally a mucin degrading bacteria.
R. bromii ferments resitant starch which is correlated with increased butyrate production downstream. The major fermentation products include acetate, H2, and CO2. The byproducts of the degradation of resistant startch are used by the bacterial species. Therefore, R. bromii supports microbiome diversity through cross-feeding. One study showed that five species, including R. bromii, were significantly more abundant in stool samples from obese individuals versus non-obese individuals. R. bromii is enriched in healthy twins versus those with food allergies. An infant study showed that depletion of R. bromii and Akkermansia muciniphila was associated with reduced butyrate and theh developmenet of atopic dermatitis.
References:
Ze, X., Duncan, S. H., Louis, P. & Flint, H. J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon microbe-microbe and microbe-host interactions. ISME J. 6, 1535–1543 (2012).
Crost, E. H. et al. Mechanistic Insights Into the Cross-Feeding of Ruminococcus gnavus and Ruminococcus bromii on Host and Dietary Carbohydrates. Front. Microbiol. 9, (2018).
Sasaki M, Schwab C, Ramirez Garcia A, Li Q, Ferstl R, Bersuch E, Akdis CA, Lauener R; CK-CARE study group; Frei R, Roduit C. The abundance of Ruminococcus bromii is associated with faecal butyrate levels and atopic dermatitis in infancy. Allergy. 2022 Aug 2;77(12):3629–40. doi: 10.1111/all.15440. Epub ahead of print. PMID: 35917214; PMCID: PMC10087690.
Bonder MJ, Tigchelaar EF, Cai X, Trynka G, Cenit MC, Hrdlickova B, Zhong H, Vatanen T, Gevers D, Wijmenga C, Wang Y, Zhernakova A. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016 Apr 21;8(1):45. doi: 10.1186/s13073-016-0295-y. PMID: 27102333; PMCID: PMC4841035.
Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017 May;66(5):863-871. doi: 10.1136/gutjnl-2015-309940. Epub 2016 Feb 4. PMID: 26848182; PMCID: PMC5531225.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559-63. doi: 10.1038/nature12820. Epub 2013 Dec 11. PMID: 24336217; PMCID: PMC3957428.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016 May 3;7(3):189-200. doi: 10.1080/19490976.2015.1134082. Epub 2016 Mar 10. PMID: 26963409; PMCID: PMC4939913.
Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio. 2019 Jan 29;10(1):e02566-18. doi: 10.1128/mBio.02566-18. PMID: 30696735; PMCID: PMC6355990.
Rangarajan AA, Chia HE, Azaldegui CA, Olszewski MH, Pereira GV, Koropatkin NM, Biteen JS. Ruminococcus bromii enables the growth of proximal Bacteroides thetaiotaomicron by releasing glucose during starch degradation. Microbiology (Reading). 2022 Apr;168(4). doi: 10.1099/mic.0.001180. PMID: 35471195.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015 Aug 11;15:100. doi: 10.1186/s12876-015-0330-2. PMID: 26261039; PMCID: PMC4531509.
Bao R, Hesser LA, He Z, Zhou X, Nadeau KC, Nagler CR. Fecal microbiome and metabolome differ in healthy and food-allergic twins. J Clin Invest. 2021 Jan 19;131(2):e141935. doi: 10.1172/JCI141935. PMID: 33463536; PMCID: PMC7810484.
Rosés C, Cuevas-Sierra A, Quintana S, Riezu-Boj JI, Martínez JA, Milagro FI, Barceló A. Gut Microbiota Bacterial Species Associated with Mediterranean Diet-Related Food Groups in a Northern Spanish Population. Nutrients. 2021 Feb 16;13(2):636. doi: 10.3390/nu13020636. PMID: 33669303; PMCID: PMC7920039.
Nieves-Ramírez ME, Partida-Rodríguez O, Laforest-Lapointe I, Reynolds LA, Brown EM, Valdez-Salazar A, Morán-Silva P, Rojas-Velázquez L, Morien E, Parfrey LW, Jin M, Walter J, Torres J, Arrieta MC, Ximénez-García C, Finlay BB. Asymptomatic Intestinal Colonization with Protist Blastocystis Is Strongly Associated with Distinct Microbiome Ecological Patterns. mSystems. 2018 Jun 26;3(3):e00007-18. doi: 10.1128/mSystems.00007-18. PMID: 29963639; PMCID: PMC6020473.
Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, Yang J, Chen L, Chen G, Qu S, Liang J, Qin L, Huang Q, Peng Y, Li Q, Wang X, Kong P, Hou G, Gao M, Shi Z, Li X, Qiu Y, Zou Y, Yang H, Wang J, Xu G, Lai S, Li J, Ning G, Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020 Oct 6;11(1):5015. doi: 10.1038/s41467-020-18414-8. PMID: 33024120; PMCID: PMC7538905.
What does it mean if your Ruminococcus bromii result is too high?
R. bromii increased on a resistant starch diet.
In a study on 360 Spanish adults with different levels of adherence to a Mediterranean diet, legume consumption was shown to enhance R. bromii.
In a population study on 156 asymptomatic Mexican adults, Blastocystis colonization was strongly correlated with an increase in R. bromii.
The abundance of Ruminococcus bromii is associated with faecal butyrate levels and atopic dermatitis in infancy.
A study on 409 type 2 diabetes Chinese patients demonstrated the reduction of R. bromii with berberine. It is thought that the hypoglycemic effect of berberine is mediated by the inhibition of secondary bile acid biotransformation by R. bromii.
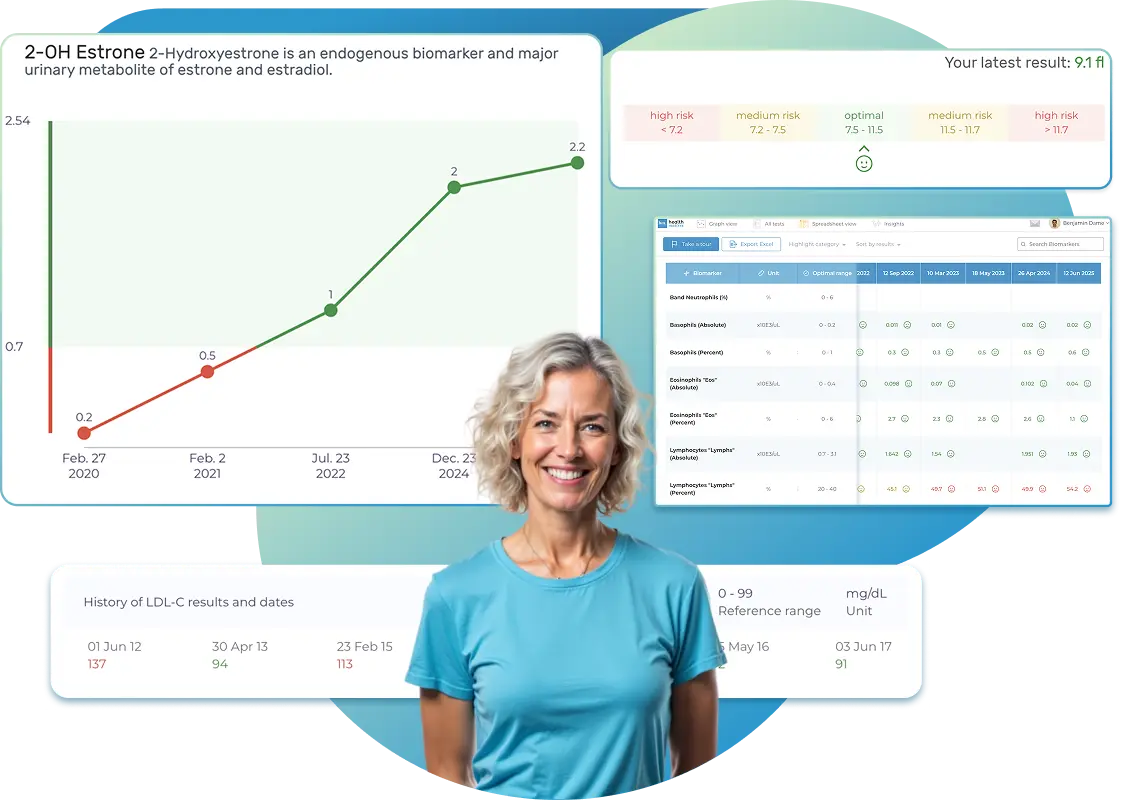
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your Ruminococcus bromii result is too low?
R. bromii decreased on a gluten-free diet in 21 healthy volunteers.
A small study on 11 healthy volunteers showed that an animal-based diet increased the abundance of bile-tolerant microorganisms (Alistipes, Bilophila and Bacteroides) and decreased the levels of Firmicutes that metabolize dietary plant polysaccharides (Roseburia, Eubacterium rectale and Ruminococcus bromii).
Oral versus IV iron supplementation in iron deficient IBD patients resulted in decreased abundances of Faecalibacterium prausnitzii, Ruminococcus bromii, Dorea spp., and Collinsella aerofaciens.
A study on 409 type 2 diabetes Chinese patients demonstrated the reduction of R. bromii with berberine. It is thought that the hypoglycemic effect of berberine is mediated by the inhibition of secondary bile acid biotransformation by R. bromii.
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
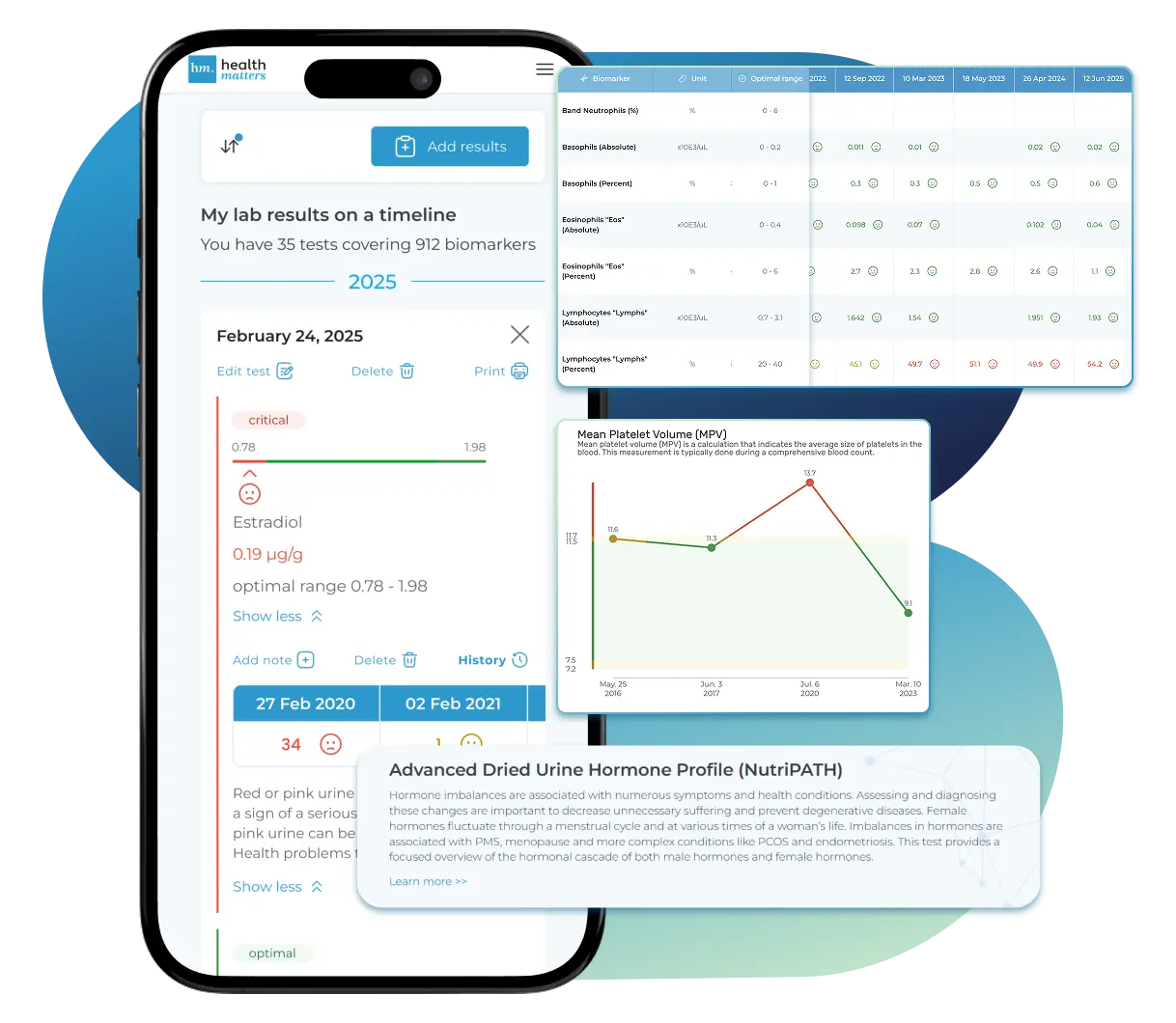
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















