Helicobacter pylori
Helicobacter pylori has been evolving with human beings for well over 50,000 years, since they migrated out of Africa. H. pylori colonization has been implicated in a variety of gastroduodenal diseases including:
- gastritis,
- gastric cancer,
- and duodenal and peptic ulcer.
H. pylori has also been detected by stool PCR in cases of:
- dyspepsia,
- abdominal pain,
- and chronic gastrointestinal symptoms.
It is infamous for its causal link to ulcers and gastric cancer, which resulted in a Nobel prize awarded to Robin Warren and Barry Marshall in 2005. However, some sources are suggesting its role, at least in part, as a commensal organism. H. pylori may protect its host from certain atopic disorders, as well as other diseases such as:
- esophageal cancer reflux,
- and obesity.
Population data shows that H. pylori virulence varies geographically. It is associated with high rates of cancer in certain regions, but not in others. The difference may lie in H. pylori’s genetics. Host immune status and acid secretion seem to be other important factors contributing to H. pylori’s colonization and pathogenesis. The H. pylori virulence factors that are most well recognized are vacA and cagA. The presence of cagA-positive H.pylori strains has been significantly associated with gastric cancer and peptic ulcer. The gene codes for a type IV secretion system which allows the bacterium to inject the cagA protein into the host cell. Once inside the host’s gastric epithelial cells, cagA can disrupt cell signaling, leading to abnormal proliferation, motility, and changes in the cytoskeleton. These changes to normal cell signaling can initiate cancer. The presence of vacA has been associated with gastric cancer, peptic ulcer, and duodenal ulcer. The vacA gene is present in all strains of H.pylori but is polymorphic, which leads to different levels of vacuolating toxin. VacA toxins interact with certain receptors on host cells, setting off a chain of events including mitochondrial damage, inhibition of T-lymphocytes, and interference of antigen presentation.
Numerous papers suggest the clinical utility of PCR testing for H. pylori. Detection of H.pylori in biopsy specimens by PCR has proven superior to other methods. It has shown sensitivity and specificity reaching that of the diagnostic “gold standard,” which is endoscopy with biopsy and urease test. H.pylori genotyping may be useful for resistant H. pylori infections that have failed to respond to triple antibiotic therapy. In one study of RT-PCR, authors stated it was a “highly accurate noninvasive method to detect H. pylori infection in stool and at the same time allows for culture-independent clarithromycin susceptibility testing.”
H. pylori may be asymptomatic and require no treatment or only supportive care to improve the intestinal mucosa and gastrointestinal lining. Information on the cagA and vacA genes may help determine whether treating a positive H. pylori result is necessary.
What does it mean if your Helicobacter pylori result is too high?
H. pylori colonization has been implicated in a variety of gastroduodenal diseases including:
- gastritis,
- gastric cancer,
- and duodenal and peptic ulcer.
H. pylori has also been detected by stool PCR in cases of:
- dyspepsia,
- abdominal pain,
- and chronic gastrointestinal symptoms.
It is infamous for its causal link to ulcers and gastric cancer, which resulted in a Nobel prize awarded to Robin Warren and Barry Marshall in 2005. However, some sources are suggesting its role, at least in part, as a commensal organism. H. pylori may protect its host from certain atopic disorders, as well as other diseases such as:
- esophageal cancer reflux,
- and obesity.
Symptoms:
Most people with H. pylori infection will never have any signs or symptoms. It's not clear why this is, but some people may be born with more resistance to the harmful effects of H. pylori.
When signs or symptoms do occur with H. pylori infection, they may include:
- An ache or burning pain in your abdomen
- Abdominal pain that's worse when your stomach is empty
- Nausea
- Loss of appetite
- Frequent burping
- Bloating
- Unintentional weight loss
Potential treatment:
H. pylori infections are usually treated with at least two different antibiotics at once, to help prevent the bacteria from developing a resistance to one particular antibiotic. Your doctor also will prescribe or recommend an acid-suppressing drug, to help your stomach lining heal. Your doctor may recommend that you undergo testing for H. pylori at least four weeks after your treatment. If the tests show the treatment was unsuccessful, you may undergo another round of treatment with a different combination of antibiotic medications.
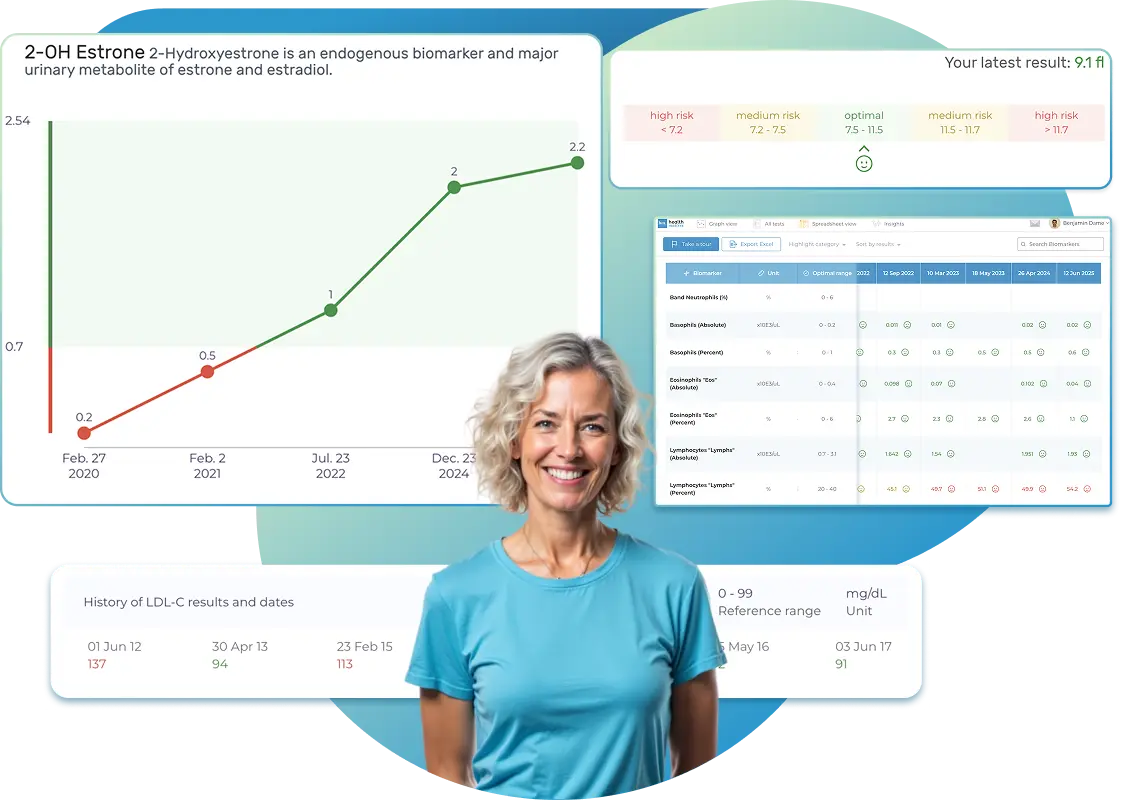
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
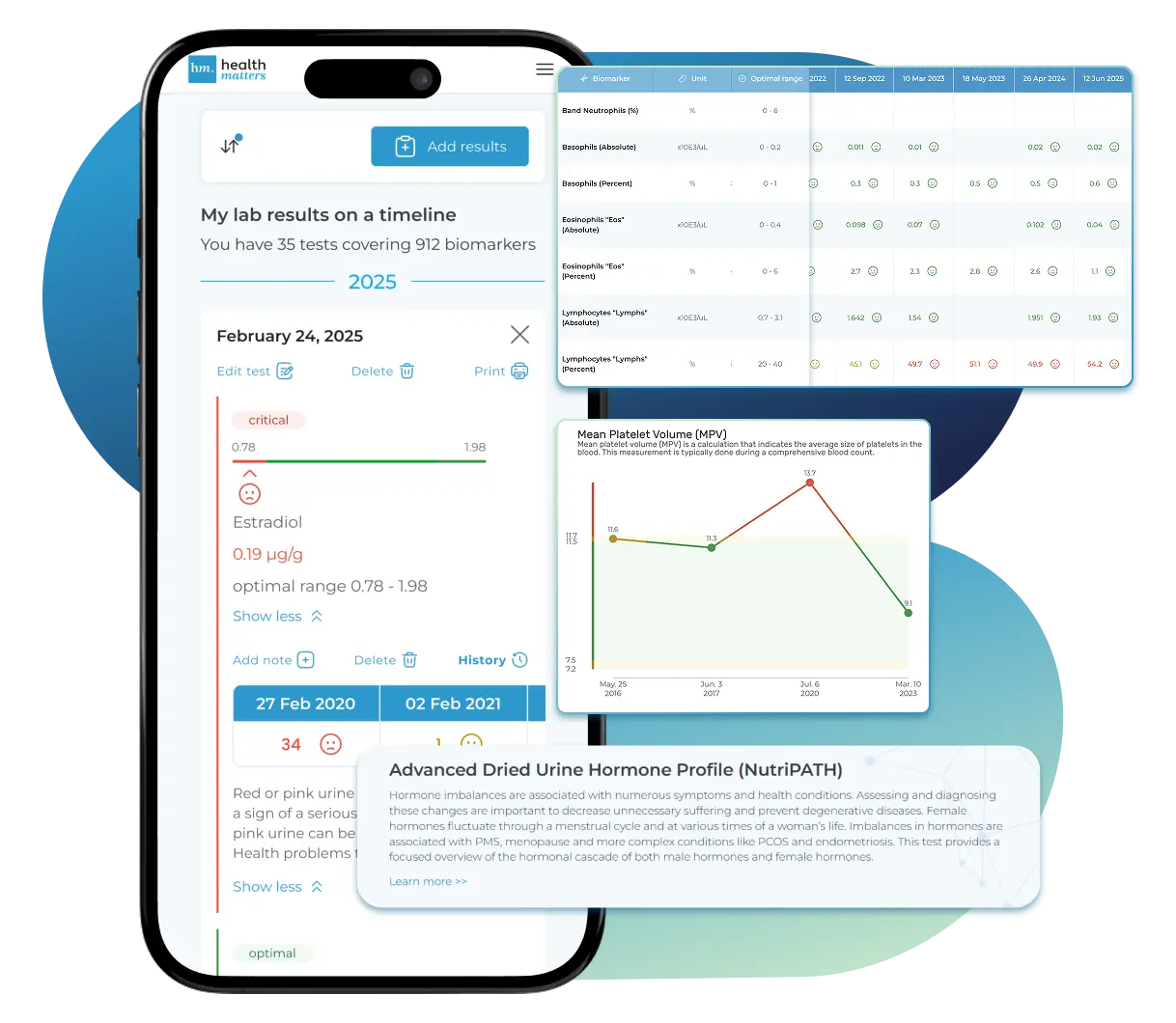
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















