The ADAMTS13 activity and inhibitor assays are useful for the diagnosis of congenital or acquired form of TTP.
ADAMTS13 is a plasma protein responsible for regulating the interaction of platelets with von Willebrand factor (VWF) and physiologic proteolytic cleavage of ultra large (UL) VWF multimers at the Tyr(1605)- Met(1606) bond in the A2 domain of VWF. Reduced or absent ADAMTS13 activity can retain UL VWF that can trigger intravascular platelet aggregation and microthrombi causing clinical symptoms or signs of thrombotic thrombocytopenic purpura (TTP).
Measurement of ADAMTS13 activity and its inhibitor is crucial in the diagnosis of TTP, potentially fatal thrombotic microangiopathy (TMA) syndrome and further differentiation of congenital (Upshaw-Schulman syndrome) versus acquired (e.g. autoimmune-related disorder) etiology.
TTP is a rare life-threatening disease with an estimated incidence of four to six cases per million, and affects more often women, particularly pregnant or postpartum women (estimated incidence of one per 25,000 pregnancies), and African-Americans. TTP is primarily diagnosed clinically, and its correct diagnosis is often very difficult.
TTP is characterized by microangiopathic hemolytic anemia including numerous schistocytes in the peripheral blood smear, thrombocytopenia, neurologic symptoms, fever, renal dysfunction, variable organ damage and ischemia, and deficient ADAMTS13 activity, usually less than 30%.
Approximately two-thirds of patients with a clinical diagnosis of idiopathic TTP will have less than 10% ADAMTS13 activity. Decreased ADAMTS13 activity in TTP is often related to autoantibodies that inhibit or clear ADAMTS13. ADAMTS13 inhibitor is observed in 44-93% of patients with severely deficient ADAMTS13 activity. Relapse occurs in 20-25% of TTP patients. Persistence of severe deficiency of ADAMTS13 activity or an inhibitor suggests high risk of relapse in symptomatic TTP.
Congenital TTP (Upshaw-Shulman syndrome) is a rare inheritable disease with an autosomal recessive pattern, and caused by genetic mutations within the ADAMTS13 gene producing non-functional ADAMTS13 protein. These patients will have severely deficient ADAMTS13 activity with high risk for recurrent episodes of TTP, and usually do not develop autoantibodies to ADAMTS13.
Acquired TTP is more common than congenital forms, and may be considered to be primary or idiopathic (the most frequent type) or associated with distinctive clinical conditions (secondary TTP). Early detection and initiation of plasma exchange is critical for better survival of patients. Quantitative measurement of ADAMTS13 activity by fluorescence energy transfer (FRET) technology will assist in the correct diagnosis of TTP.
Quantitation of the ADAMTS13 activity level will be also useful to distinguish patients with TTP from other thrombocytopenic conditions such as hemolytic uremic syndrome (HUS), immune thrombocytopenic purpura (ITP) or heparininduced thrombocytopenia (HIT).
What does it mean if your Adamts13 Activity result is too high?
Recent plasma exchange or immunosuppressive therapy may raise the observed activity levels.
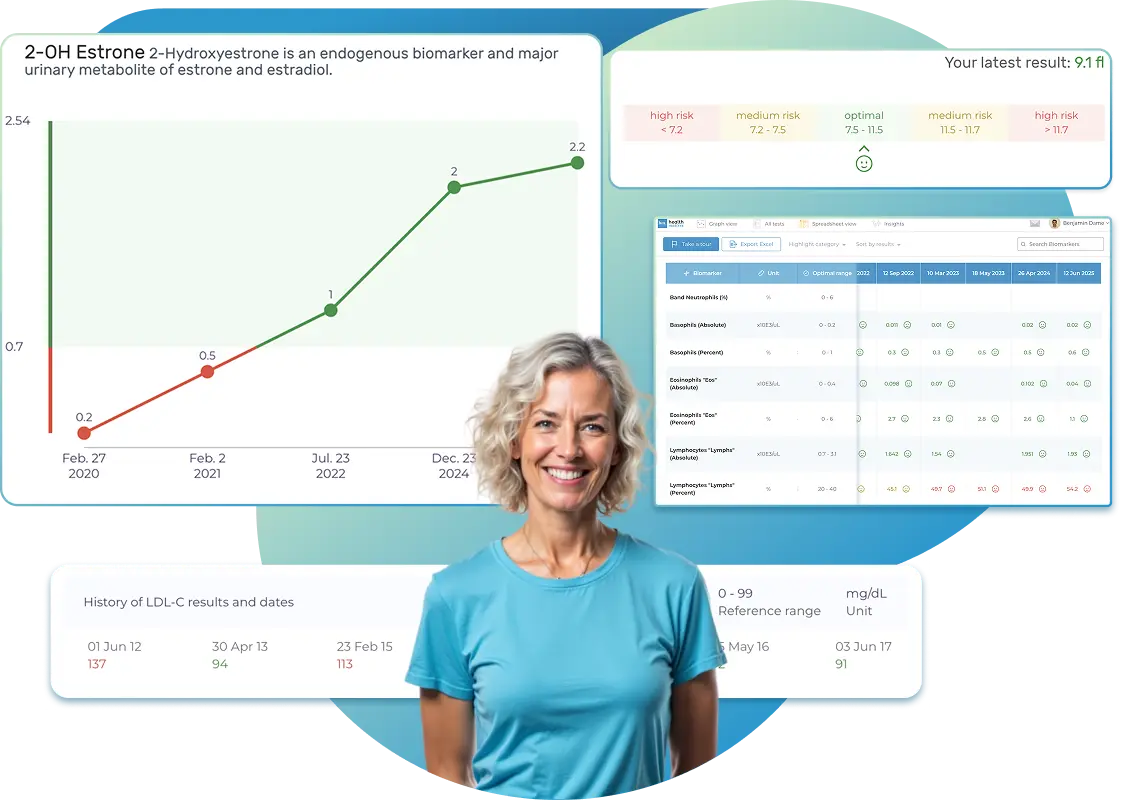
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your Adamts13 Activity result is too low?
Activity levels below 0.10 IU/mL are seen in acquired and hereditary thrombotic thrombocytopenic purpura (TTP). Not all patients with TTP will exhibit low levels of ADAMTS13 activity with this assay, i.e., post bone marrow transplantation, drug-induced TTP, and mutations of ADAMTS13 at the CUB domain.
Mild decreases in ADAMTS13 activity are seen in a wide variety of conditions including metastatic cancer, neonates, serious infections and cirrhosis of the liver.
Decreased ADAMTS13 activity (equal to or less than 68%) can be observed in idiopathic (autoimmune-related) TTP, TMA syndrome, congenital ADAMTS13 deficiency (UpshawSchulman syndrome) and secondary to other clinical conditions such as HUS, ITP, solid organ or bone marrow transplantation, sepsis, DIC, HIV infection, inflammation, bloody diarrhea, liver disease, pregnancy, malignancy, or certain drug effects (e.g., clopidogrel, cyclosporin, mitomycin C, ticlopidine, tacrolimus, etc).
1. If ADAMTS13 activity is decreased (less than 30%), ADAMTS13 inhibitor assay is further evaluated for titration of inhibitor unit. a) Greater than 0.4 Inhibitor Unit is diagnostic of idiopathic TTP. b) Less than 0.4 Inhibitor Unit suggests autoantibody assay (send-out test) for further differentiation of congenital or acquired TTP. Autoantibody, usually IgG by ELISA method, is elevated in idiopathic TTP. However, it can be detected in other immune-mediated disorders, and some healthy individuals (10-15%). If autoantibody is low, ADAMTS13 sequencing for genetic mutation is suggested to rule out congenital TTP.
2. Severely decreased ADAMTS13 activity (less than 5-10%) is considered as a relatively specific laboratory finding for the clinical diagnosis of congenital and acquired idiopathic TTP.
3. Mildly decreased ADAMTS13 activity (30-67%) is often observed in TTP patients secondary to other clinical conditions, and unlikely related to idiopathic (autoimmunerelated) TTP. However, if there is a strong clinical suspicion of TTP, inhibitor assay can be performed.
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
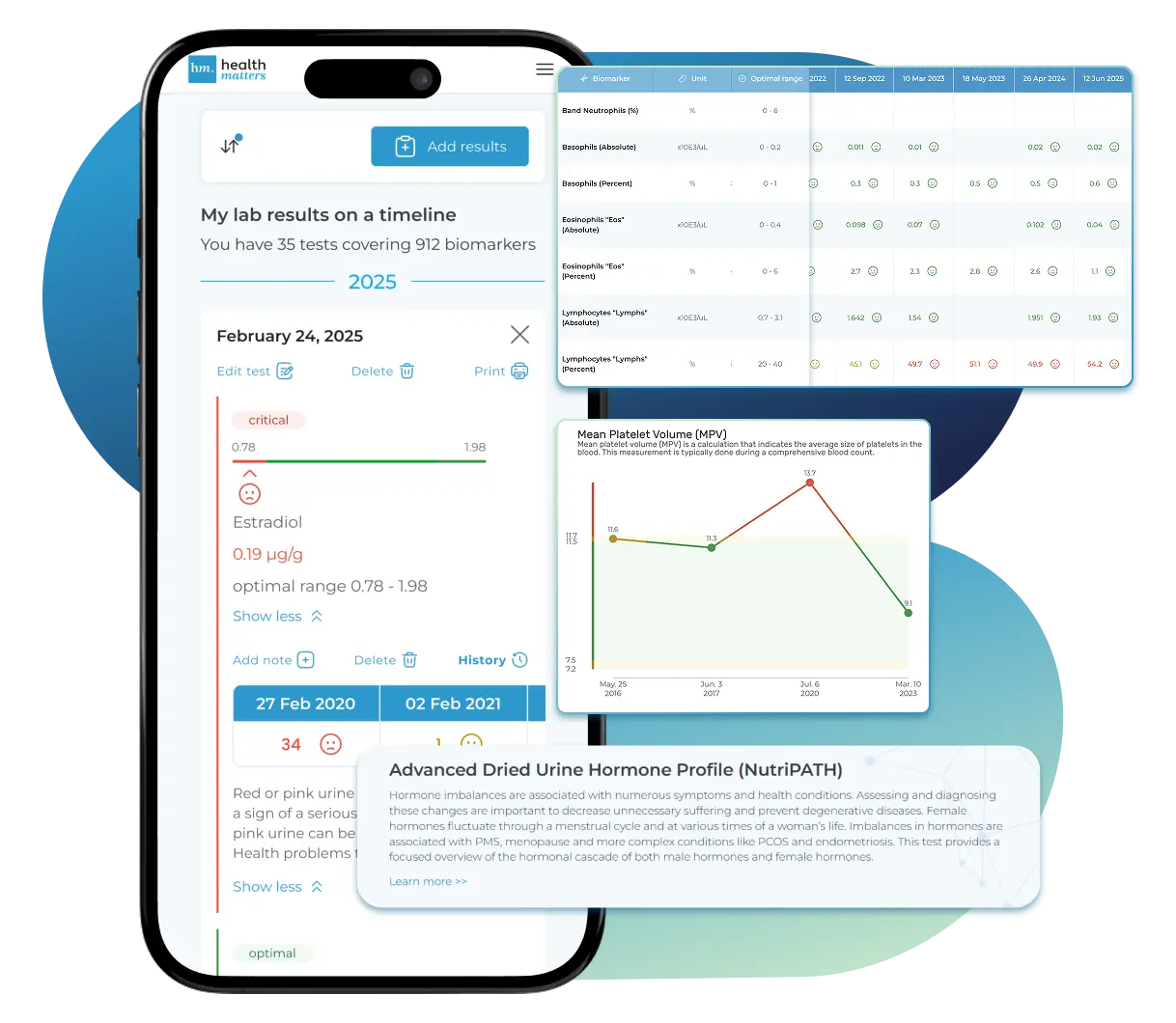
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















