CD25 is the receptor for IL2 and is expressed on activated T cells, B cells, and macrophages. CD25 is expressed in certain types of B-cell lymphoma (hairy cell leukemia) and T-cell lymphoma (adult T-cell lymphoma/leukemia [ATLL]).
The interleukin-2 receptor is designated CD25. Originally isolated from T-lymphocytes, it is now known to be expressed on hairy cell leukemia and adult T-cell leukemia/lymphoma, classical Hodgkin lymphoma, and a subset of other peripheral T-cell lymphomas.
General information:
Lymphocytes in peripheral blood (circulation) are heterogeneous and can be broadly classified into T cells, B cells, and natural killer (NK) cells.
There are various subsets of each of these individual populations with specific cell-surface markers and function.
This assay provides absolute (cells/mcL) and relative (%) quantitation for the main categories of T cells, B cells, and NK cells, in addition to a total lymphocyte count (= CD45+).
- Each of these lymphocyte subpopulations have distinct effector and regulatory functions and are maintained in homeostasis under normal physiological conditions.
- Each of these lymphocyte subsets can be identified by a combination of one or more cell surface markers.
T-cell:
The CD3 antigen is a pan-T-cell marker, and T cells can be further divided into 2 broad categories, based on the expression of CD4 or CD8 coreceptors.
B cells:
B cells can be identified by expression of CD19.
NK cells:
NK cells are typically identified by the coexpression of CD16 and CD56.
Summary:
Total T-cells (CD3+)
B-cells (CD3-/CD19+)
Natural killer (NK) cells (CD3-/CD56+CD16+)
Helper/inducer (CD3+/CD4+) T-cell subset
cytotoxic/suppressor (CD3+/CD8+) T-cell subset
Total CD45+ lymphocyte count
CD4:CD8 ratio
-----------------------------
The absolute counts of lymphocyte subsets are known to be influenced by a variety of biological factors, including hormones, the environment, and temperature. The studies on diurnal (circadian) variation in lymphocyte counts have demonstrated progressive increase in CD4 T-cell count throughout the day, while CD8 T cells and CD19+ B cells increase between 8:30 a.m. and noon with no change between noon and afternoon. NK-cell counts, on the other hand, are constant throughout the day.
Circadian variations in circulating T-cell counts have been shown to be negatively correlated with plasma cortisol concentration. In fact, cortisol and catecholamine concentrations control distribution and, therefore, numbers of naive versus effector CD4 and CD8 T cells.
It is generally accepted that lower CD4 T-cell counts are seen in the morning compared to the evening and during summer compared to winter. These data, therefore, indicate that timing and consistency in timing of blood collection is critical when serially monitoring patients for lymphocyte subsets.
Abnormalities in the number and percent of T (CD3, CD4, CD8), B (CD19), and NK (CD16+CD56) lymphocytes have been described in a number of different disease conditions.
In patients who are infected with HIV, the CD4 count is measured for AIDS diagnosis and for initiation of antiviral therapy. The progressive loss of CD4 T lymphocytes in patients infected with HIV is associated with increased infections and complications. The Public Health Service has recommended that all patients who are HIV-positive be tested every 3 to 6 months for the level of CD4 T lymphocytes.
Lymphocyte subset quantitation is also very useful in the evaluation of patients with primary immunodeficiencies of all ages, including follow-up for newborn screening for severe combined immunodeficiency and immune monitoring following immunosuppressive therapy for transplantation, autoimmunity, or any other relevant clinical condition where immunomodulatory treatment is used.
It is also helpful as a preliminary screening assay for gross quantitative anomalies in any lymphocyte subset, whether related to malignancies or infection.
The 2008 guidelines for diagnosis and treatment of Chronic Lymphocytic Leukemia (CLL) from the International Workshop on Chronic Lymphocytic Leukemia recommends changing the diagnostic criteria for CLL from an absolute lymphocyte count greater than 5 x 10(9)/L to a circulating B-cell count greater than 5 x 10(9)/L(8,9) previously defined in the 1996 National Cancer Institute (NCI) guidelines for CLL. This flow cytometric assay enables accurate quantitation of circulating B cells using a single platform technology with absolute quantitation through the use of flow cytometry beads.
Sources:
https://www.mayocliniclabs.com/test-catalog/Overview/9336#Clinical-and-Interpretive
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/t-cell
https://www.rcpa.edu.au/Manuals/RCPA-Manual/Pathology-Tests/L/Lymphocyte-immunophenotyping
References:
Milioglou I, Kalaitzidou I, Ladomenou F. Interpretation of lymphocyte subset counts by the general pediatrician. Pediatr Int. 2019 Jan;61(1):16-22. doi: 10.1111/ped.13701. Epub 2018 Dec 10. PMID: 30248214.
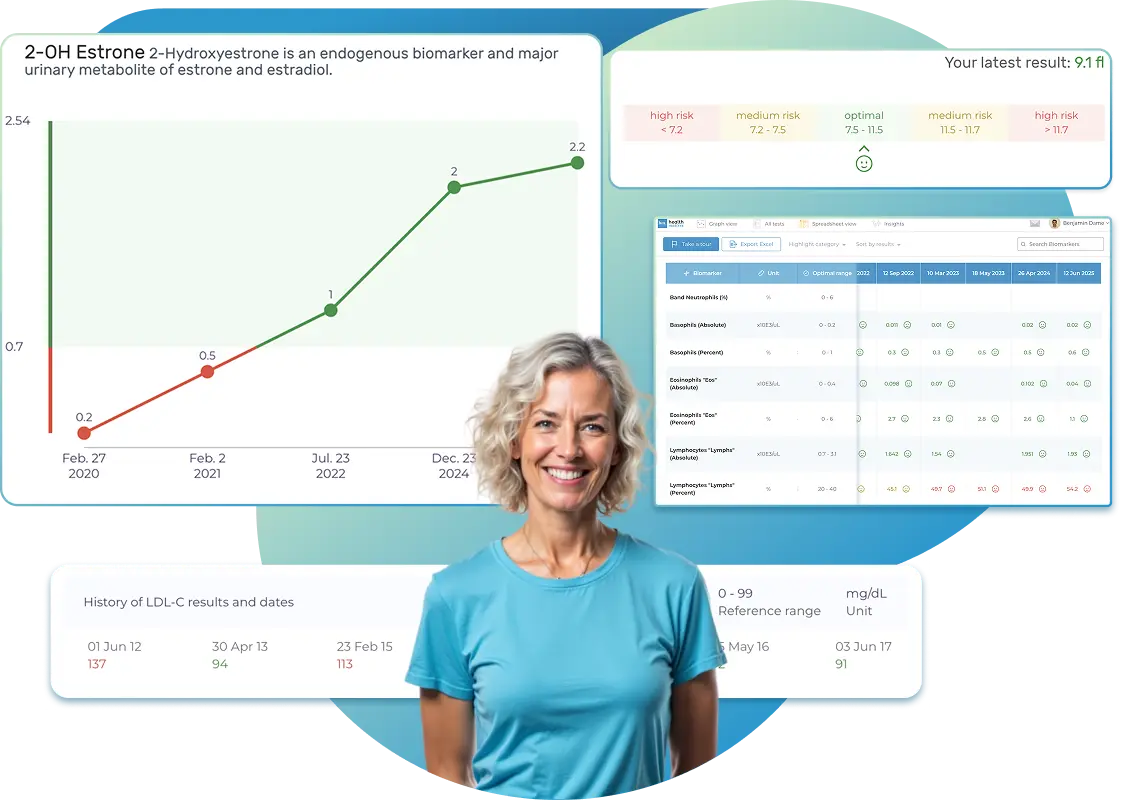
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
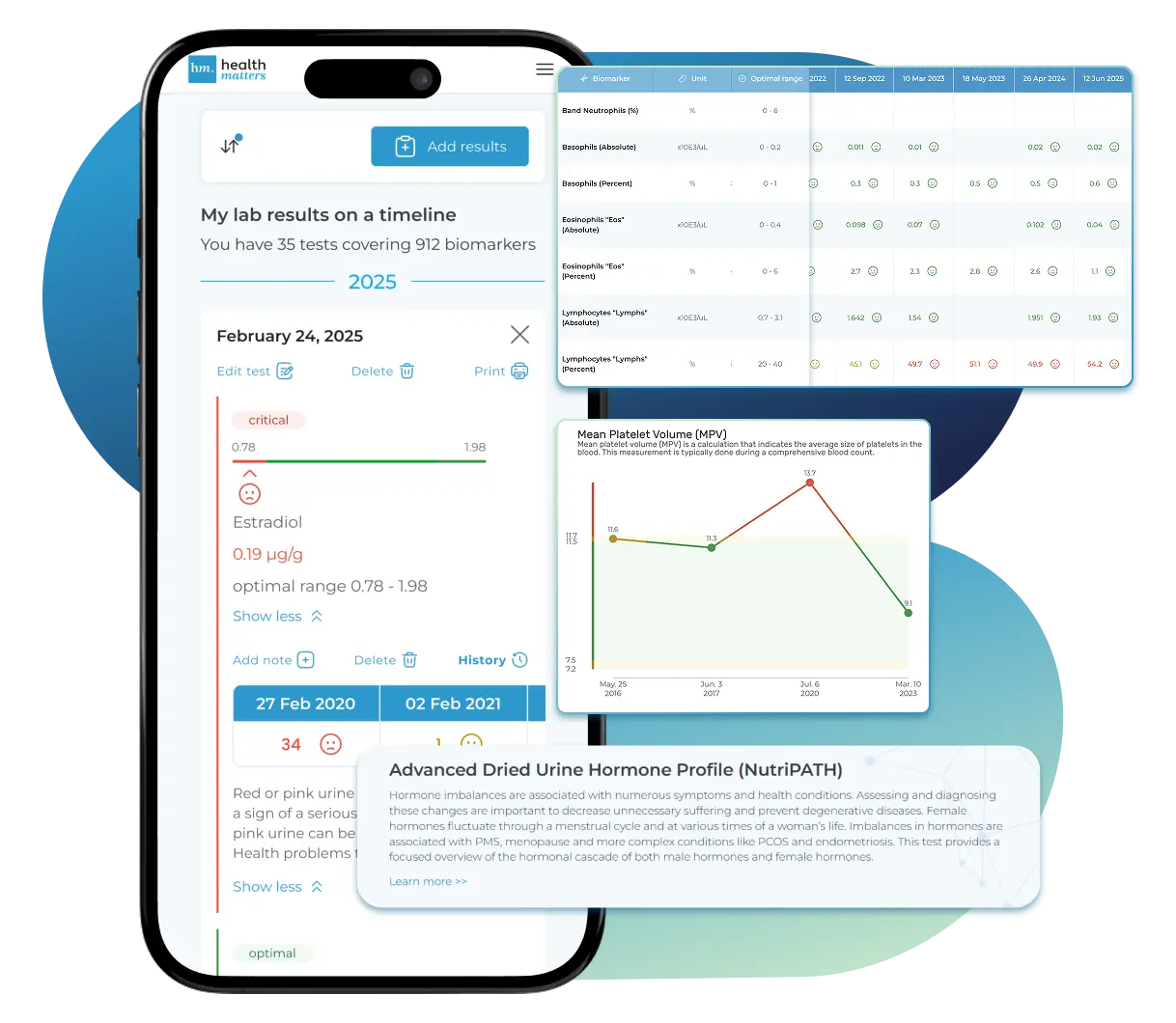
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















