16-OH-E1 Postmenopausal
Both 16a-OHE1 and 4-OHE1 have been referred to as the “bad” estrogens.
- This estrogen metabolite is a more potent estrogen than the other two (2-OH and 4-OH). If this value is elevated, we may see estrogen dominance.
- Estrogen is also metabolized through the ’16’ pathway. We need this route however it should be used significantly less than the 2-OH pathway. Phase 1 in the 16-Hydroxyestrone pathway is also hydroxylation. Phase 2 is a reduction phase that produces Estriol which is a weak estrogen.
16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-trien-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-OH-E1, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.
Synthetic and Natural Estrogens:
Estrone (E1), estradiol (E2) and estriol (E3) are the three estrogens. Synthetic estrogen (premarin) is made up of E1 and E2 estrogen. E1 is the main estrogen that the body makes post menopausally, and most researchers believe that high E1 levels increase the risk of breast cancer.
Estrogen Metabolism:
The metabolism of estrogen in women changes after menopause. The body metabolizes estrogen into two major pathways and one minor. The two major are 2 and 16-hydroxyeone (2-OH and 16 OH, respectively). The minor pathway is 4-hydroxyestrone (4-OH). 2-OH is the good metabolite. 2-OH does not stimulate cell growth and it blocks the action of stronger estrogens that may be carcinogenic.
16-OH has a significantly stronger estrogenic activity, and studies show that it may increase the risk of breast cancer.
4-OH is also not desirable as it may directly damage DNA and cause mutations, thus it is thought to promote cancer development. Premarin breaks down exclusively into 4-hydroxyestrogen.
What does it mean if your 16-OH-E1 Postmenopausal result is too high?
- Higher levels of the 16 pathway are associated with breast cancer, obesity, hypothyroidism, pesticide toxicity (organochlorines), high Omega-6 fatty acids, and inflammatory cytokines.
- 16-OH-E1 has been shown to encourage tumor development
Lowering levels of 16aOH-E1 have been achieved via indole-3-carbinol or one of its metabolites, di-indol methane (DIM). Soy and flax meal have also been shown to lower 16aOH-E1 levels.
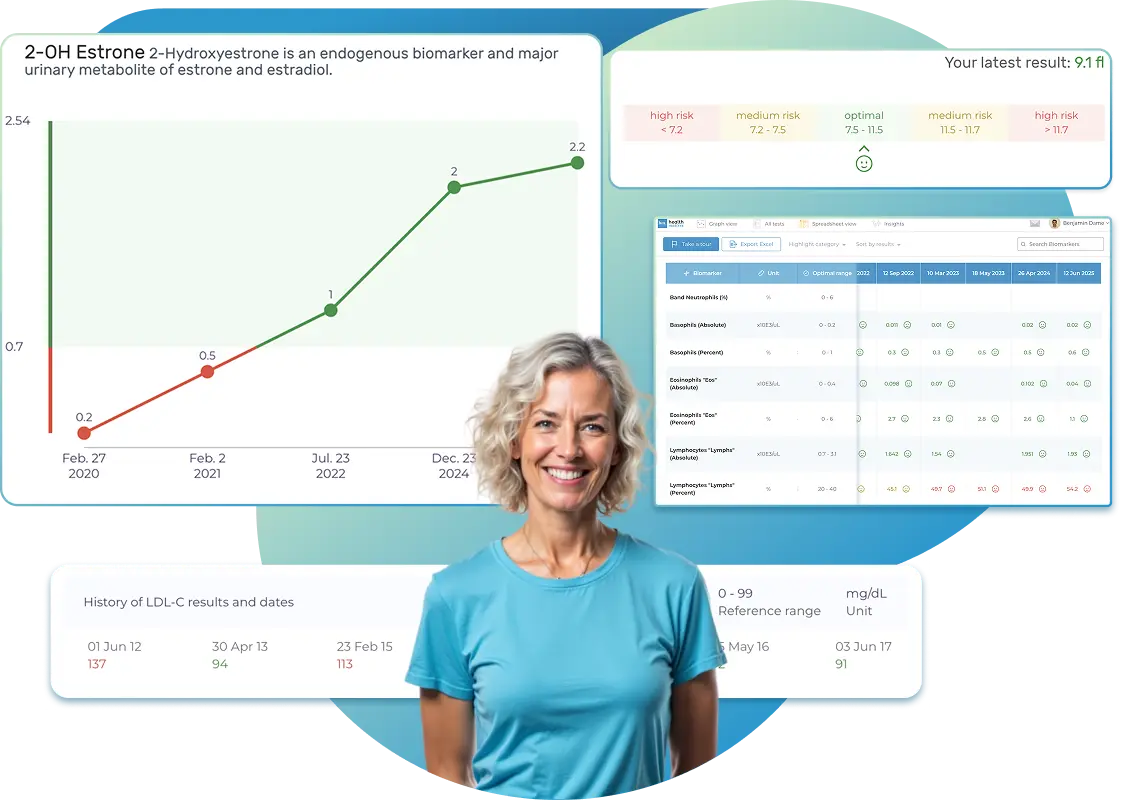
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
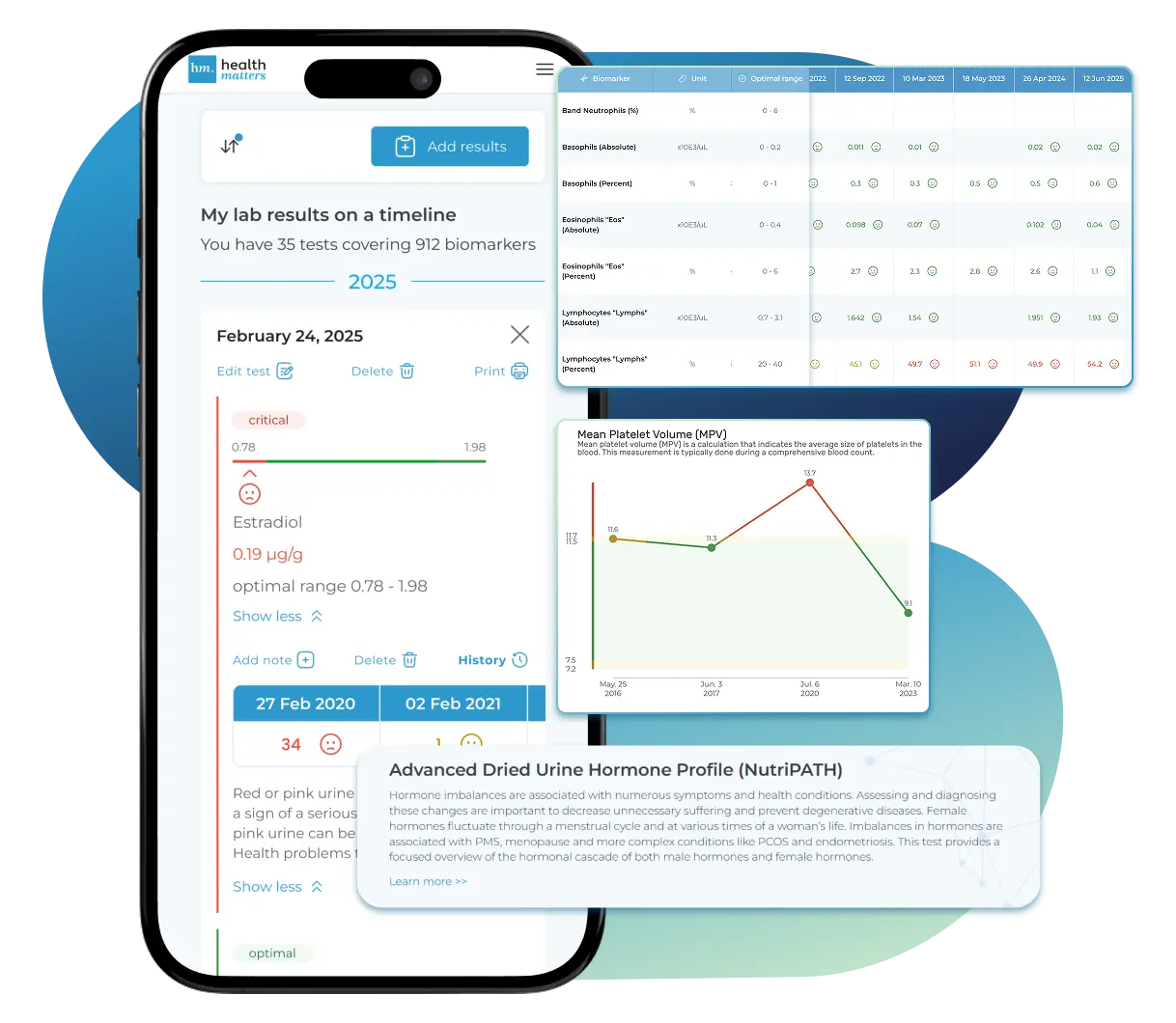
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your 16-OH-E1 Postmenopausal result is too low?
Low levels of the “bad estrogen” are typically considered beneficial.
16-OH-E1 is the immediate precursor to the weak estrogen, estriol (E3). Please also note that 16-OH-E1 is important for maintaining bone mineral density. Excessive reduction of 16-OH-E1 may reduce bone formation and increase oxidant stress.
If 16-OH-E1 is low and 2-OH-E1 is low, then total estrogen production may be low. Address underlying causes of low hormone production, as needed.
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















