16-OH-E1
Both 16a-OHE1 and 4-OHE1 have been referred to as the “bad” estrogens.
- This estrogen metabolite is a more potent estrogen than the other two (2-OH and 4-OH). If this value is elevated, we may see estrogen dominance.
- Estrogen is also metabolized through the ’16’ pathway. We need this route however it should be used significantly less than the 2-OH pathway. Phase 1 in the 16-Hydroxyestrone pathway is also hydroxylation. Phase 2 is a reduction phase that produces Estriol which is a weak estrogen.
16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-trien-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol. It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-OH-E1, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer. Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.
Synthetic and Natural Estrogens:
Estrone (E1), estradiol (E2) and estriol (E3) are the three estrogens. Synthetic estrogen (premarin) is made up of E1 and E2 estrogen. E1 is the main estrogen that the body makes post menopausally, and most researchers believe that high E1 levels increase the risk of breast cancer.
Estrogen Metabolism:
The metabolism of estrogen in women changes after menopause. The body metabolizes estrogen into two major pathways and one minor. The two major are 2 and 16-hydroxyeone (2-OH and 16 OH, respectively). The minor pathway is 4-hydroxyestrone (4-OH). 2-OH is the good metabolite. 2-OH does not stimulate cell growth and it blocks the action of stronger estrogens that may be carcinogenic.
16-OH has a significantly stronger estrogenic activity, and studies show that it may increase the risk of breast cancer.
4-OH is also not desirable as it may directly damage DNA and cause mutations, thus it is thought to promote cancer development. Premarin breaks down exclusively into 4-hydroxyestrogen.
What does it mean if your 16-OH-E1 result is too high?
- Higher levels of the 16 pathway are associated with breast cancer, obesity, hypothyroidism, pesticide toxicity (organochlorines), high Omega-6 fatty acids, and inflammatory cytokines.
- 16-OH-E1 has been shown to encourage tumor development
Lowering levels of 16aOH-E1 have been achieved via indole-3-carbinol or one of its metabolites, di-indol methane (DIM). Soy and flax meal have also been shown to lower 16aOH-E1 levels.
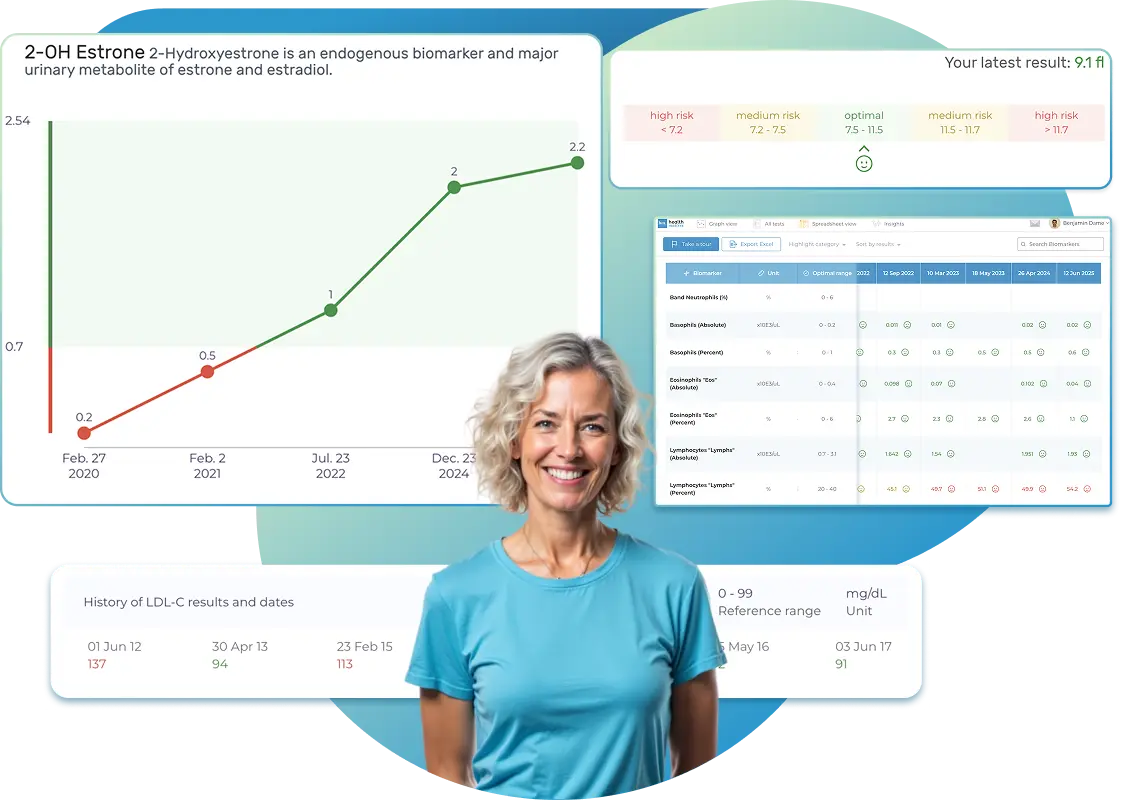
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
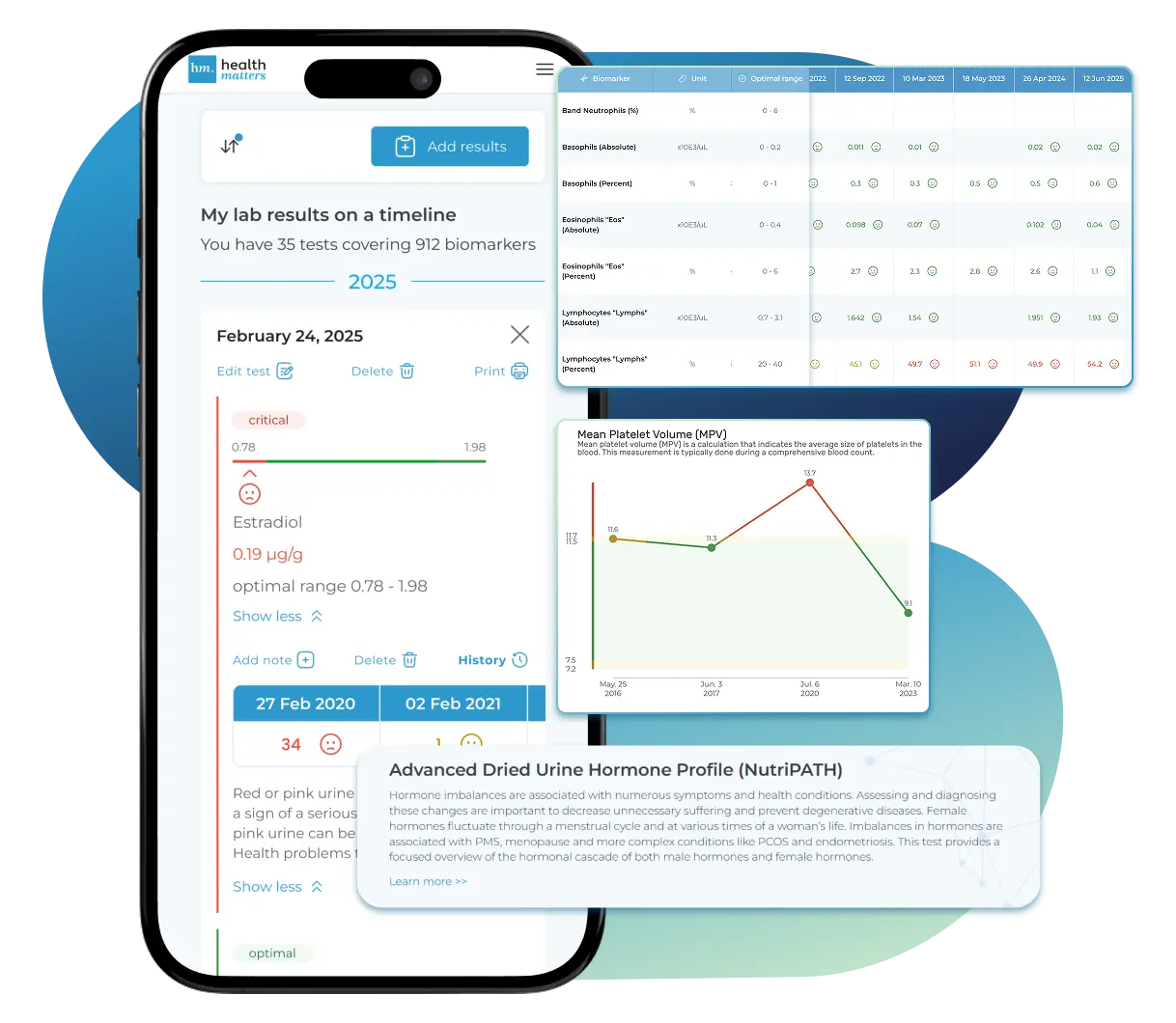
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
What does it mean if your 16-OH-E1 result is too low?
Low 16-OH-E1 on a DUTCH Test: What Does It Mean?
What is 16-OH-E1?
16α-Hydroxyestrone (16-OH-E1) is one of the three major estrogen metabolites in the body, formed when estrone (E1) is metabolized through the 16-hydroxy pathway. This pathway leads to the production of estriol (E3), the weakest of the three primary estrogens (estradiol, estrone, and estriol).
16-OH-E1 is often considered a proliferative estrogen, meaning it has stronger estrogenic effects compared to 2-OH-E1 (which is protective and anti-proliferative) and 4-OH-E1 (which can be more reactive and potentially damaging if not properly detoxified).
What Do Low 16-OH-E1 Levels Mean?
1. Low Estrogen Metabolism via the 16-Hydroxy Pathway
Since 16-OH-E1 is a downstream estrogen metabolite, low levels may suggest reduced estrogen metabolism through this pathway. This could be due to:
- Low overall estrogen production (look at estrone (E1) and estradiol (E2) levels for confirmation).
- A genetic tendency toward favoring the 2-OH or 4-OH pathways instead.
- Liver or gut issues affecting estrogen metabolism and detoxification.
2. Low Estriol (E3) Production
Since 16-OH-E1 is a precursor for estriol (E3), low levels may contribute to:
- Reduced estriol levels, which is especially relevant for pregnancy and vaginal health.
- Increased vulnerability to vaginal dryness, irritation, and urinary tract issues, particularly in postmenopausal women.
3. Possible Hypothyroidism or Impaired Detoxification
- Low thyroid function (hypothyroidism) has been linked to decreased 16-OH-E1 metabolism.
- Liver congestion, poor detoxification, or gut dysbiosis may also reduce estrogen metabolism through the 16-hydroxy pathway.
4. Reduced Estrogenic Stimulation of Tissues
Because 16-OH-E1 is more estrogenic than 2-OH-E1, lower levels may lead to:
- Lower overall estrogenic activity in tissues such as breast, bones, and the uterus.
- Lower bone density and weaker connective tissue integrity in some cases, particularly in postmenopausal women.
Potential Symptoms of Low 16-OH-E1
Individuals with low 16-OH-E1 levels may experience symptoms of low estrogen metabolism or reduced estrogenic effects, including:
- Dry skin, vaginal dryness, or thinning of vaginal tissues
- Weaker bones (osteopenia/osteoporosis risk, especially postmenopause)
- Reduced libido and sexual dysfunction
- Low energy, poor mood, or brain fog
- Possible thyroid dysfunction symptoms (fatigue, hair thinning, weight gain)
Key Factors That Can Contribute to Low 16-OH-E1
1. Low Overall Estrogen Levels
- If estrone (E1) and estradiol (E2) are low, 16-OH-E1 may also be low due to low estrogen production overall.
2. Genetic Differences in Estrogen Metabolism
- Some individuals naturally favor the 2-hydroxy or 4-hydroxy estrogen pathways, which could result in low 16-OH-E1 levels.
3. Poor Liver Function or Detoxification
- The liver is responsible for estrogen metabolism, and liver congestion, poor bile flow, or a high toxic burden can alter estrogen processing.
- Glucuronidation and sulfation pathways in the liver and gut microbiome influence estrogen metabolism.
4. Gut Dysbiosis and Impaired Estrobolome Function
- The estrobolome (the collection of gut bacteria that metabolizes estrogens) plays a role in estrogen recycling and excretion.
- Dysbiosis, constipation, or poor gut health can alter estrogen metabolism and reduce the 16-OH pathway activity.
5. Low Thyroid Function (Hypothyroidism)
- Thyroid hormones influence estrogen metabolism, and hypothyroidism has been linked to low 16-OH-E1 levels.
- If you suspect thyroid issues, check TSH, free T3, and free T4.
Next Steps: How to Support Healthy 16-OH-E1 Levels
1. Assess Overall Estrogen Levels
- Check estrone (E1) and estradiol (E2) levels to determine if low estrogen production is contributing to low 16-OH-E1.
- Consider hormonal support strategies (if needed) under medical guidance.
2. Support Liver Detoxification
- Eat cruciferous vegetables (broccoli, cauliflower, Brussels sprouts) to support healthy estrogen metabolism.
- Ensure adequate intake of B vitamins (B6, B12, folate), magnesium, and glutathione precursors to aid phase I and phase II liver detoxification.
- Reduce alcohol and processed foods that burden the liver.
3. Improve Gut Health and Estrogen Metabolism
- Increase fiber intake (flaxseeds, vegetables, whole grains) to support healthy estrogen clearance.
- Support a balanced microbiome with probiotics and fermented foods.
- Address constipation, as sluggish digestion can lead to estrogen recycling and imbalanced estrogen metabolism.
4. Consider Thyroid Function
- If symptoms of fatigue, cold intolerance, dry skin, or hair thinning are present, assess thyroid hormones (TSH, free T3, free T4, reverse T3, and thyroid antibodies).
5. Lifestyle Adjustments
- Engage in regular physical activity, as exercise improves estrogen metabolism and hormone balance.
- Manage chronic stress, since prolonged stress can alter HPA axis function, impacting estrogen metabolism.
Final Thoughts
Low 16-OH-E1 levels on a DUTCH test may indicate low overall estrogen metabolism, poor liver detoxification, thyroid dysfunction, or gut imbalances. Since 16-OH-E1 is a precursor to estriol (E3), lower levels may impact bone health, vaginal health, and estrogenic stimulation of tissues.
By evaluating overall estrogen levels, supporting detoxification pathways, and addressing potential thyroid or gut issues, you can optimize hormonal balance and estrogen metabolism for better health.
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















