Aflatoxin Group: (B1, B2, G1, G2)
Aflatoxins were initially isolated and identified as the causative toxins in Turkey X disease (necrosis of the liver) in 1960 when over 100,000 turkeys died in England (Asao, 1963). They are the most studied mycotoxins with over 5,000 research papers published through 2002. There are four generally recognized Aflatoxins designated B1, B2, G1 and G2 . The metabolites, M1 & M2, are found in milk (Thirumala-Devi et al (2002).
The order of toxicity is B1 greater than G1, greater than G2, greater than B2. (IARC, 1976). However, Aflatoxin B1 is the major mycotoxin produced by most species under culture conditions (Ciegler & Bennet, 1980). Because of this and its toxicity, B1 is the most frequently studied of the four.
Aflatoxins are produced by different species of Aspergillus, particularly flavus, oryzae, fumigatus and parasiticus, as well as members of the genera Penicillium (El-Naghy et al, 1991; Searle 1976; Aflatoxins 2002). Strains of Aspergillus flavus and parasiticus produce mycotoxins under favorable conditions.
Aflatoxins can contaminate corn, cereals, sorghum, peanuts and other oil-seed crops. Thus, food contamination by this group of mycotoxins has been implicated in both animal and human Aflatoxicosis.
Aflatoxins are carcinogenic to humans and animals. Overall summary evaluation of carcinogenic risk to humans is Group 1 (IARC, 1976; Searle, 1976; Dominguez-Malagon & Gaytan-Graham (2001).
Aflatoxin B1 is a potent liver carcinogen in a variety of experimental animals. It causes liver tumors in mice, rats, fish, marmosets, tree shrews and monkeys following administration by various routes. Types of cancers described in research animals include hepatocellular carcinoma (rats), colon and kidney (rats), cholangiocellular cancer (hamsters), lung adenomas (mice), and osteogenic sarcoma, adenocarcinoma of the gall bladder and carcinoma of the pancreas (monkeys) (IARC, 1976).
In humans, Aflatoxin B1 has been linked to hepatocellular carcinoma from three studies reported in the medical literature, as follows:
An increased incidence (10 % excess) of hepatocellular carcinoma was reported in the southeastern portion of the U.S. in areas of high daily intake of Aflatoxin B1. The daily intake of B1 in the southeastern subjects was 13-197 ug/kg body weight as compared to those in northern and western areas with a daily intake of 0.2-0.3 ug/kg body weight (IARC, 1987).
In China, a strong correlation between the intake of peanut, peanut oil and corn and increased mortality rates for liver cancer were reported in five groups of inhabitants from four villages. The mortality rates were 125, 97.40, 41.65, 24.01 and 1.05, respectively. The median intake of aflatoxin B1 for each group was 6.05, 6.36, 2.69, 1.83 and 0 ug/day. The median daily urine concentrations of M1 metabolite were 16.46, 8, 29, 4.78 and 1.21 ng/person. A significant correlation was found between the mortality rates of primary liver cancer and intake of aflatoxin B1. Further, analysis of M1 in the urine can be used as an index for human exposure to aflatoxin B1 in an epidemiological study (Aflatoxins, 2002).
Cancer in 67 men who had inhaled particles contaminated with aflatoxin were reported in an 11-year follow up study. They worked in a mill crushing peanuts and other oil seeds. Two of the men developed fatal liver disease, while eleven developed cancers of various organs. The 13 men had inhaled doses estimated to be between 160 to 395 ug/cubic meter/man/wk. In 55 matched control men, 4 developed cancer and none died from liver disease. The excess cancers in this study was not significant, however the number of subjects was insufficient to exclude a significant positive correlation (Aflatoxins, 2002; IARC,1976).
Finally, it was reported that in hepatocellular carcinoma cases exposed to aflatoxin B1, mutation of p53 gene is fixed at codon 249 third base and takes the form of G to T transversion. It appears from the reported observations that it is a definite marker of mutation, which is induced by aflatoxin B1 mutagen and is applicable for molecular epidemiology survey of sufferers of aflatoxin B1 exposure among hepatocellular carcinoma cases (Deng & Ma, 1998).
What does it mean if your Aflatoxin Group: (B1, B2, G1, G2) result is too high?
Aflatoxins: Potent Environmental Carcinogens
Aflatoxins are among the most toxic and carcinogenic substances found in the environment. These mycotoxins are primarily produced by Aspergillus species and commonly contaminate a variety of food products, including beans, corn, rice, tree nuts, wheat, milk, eggs, and meat. They can even be detected in human tissues, particularly in cases of lung aspergilloma.
Health Effects of Aflatoxins
Susceptibility to aflatoxin toxicity depends on multiple factors, including age, sex, diet, and genetic makeup. Aflatoxins are known to cause:
-
Liver damage and hepatocellular carcinoma (liver cancer)
-
Mental impairment, abdominal pain, hemorrhaging, coma, and death in severe cases
-
Encephalopathy and Reye’s syndrome, particularly in children
-
Immunotoxicity, including reduced leukocyte proliferation and elevated alpha tumor necrosis factor (TNF-α) levels
-
Birth and neonatal risks, such as developmental toxicity, increased susceptibility to infections, jaundice, and poor vaccine response
Clinical signs of aflatoxicosis may include:
-
Non-itchy macular rash
-
Headache and gastrointestinal distress
-
Lower limb edema
-
Anemia and jaundice
Exposure in Humans and Animals
Aflatoxins have been found in:
-
Human cord blood and breast milk
-
Cow’s milk, dairy products, and infant formula
-
Tissues of both humans and animals, indicating widespread food chain contamination
Animal studies have confirmed immunosuppressive effects across multiple species, and exposure during gestation may impact fetal development.
Risk Factors and Genetic Susceptibility
Aflatoxin B1 has been shown to induce mutations in both nuclear and mitochondrial DNA. Individuals with specific genetic polymorphisms—such as GSTM1 null, mEH heterozygote, or CYP3A5 mutations—may have increased levels of albumin-aflatoxin adducts, indicating higher vulnerability to toxicity.
Mitigation and Treatment Strategies
-
Supportive care includes hydration to prevent dehydration and manage systemic effects.
-
Nutritional support: Diets rich in carrots, parsnips, celery, and parsley may reduce aflatoxin’s carcinogenic effects.
-
Clay binders like bentonite may reduce intestinal absorption of aflatoxins from contaminated food.
-
Supplementation with chlorophyllin, zinc, and vitamins A, C, and E has shown potential in reducing toxicity.
-
Pharmacologic support: Oltipraz enhances detoxification by promoting glutathione conjugation and inhibiting harmful P450-mediated metabolism.
Combined Mycotoxin Toxicity
Aflatoxin toxicity can be exacerbated by co-exposure to other mycotoxins such as ochratoxin and zearalenone, highlighting the importance of comprehensive food safety monitoring.
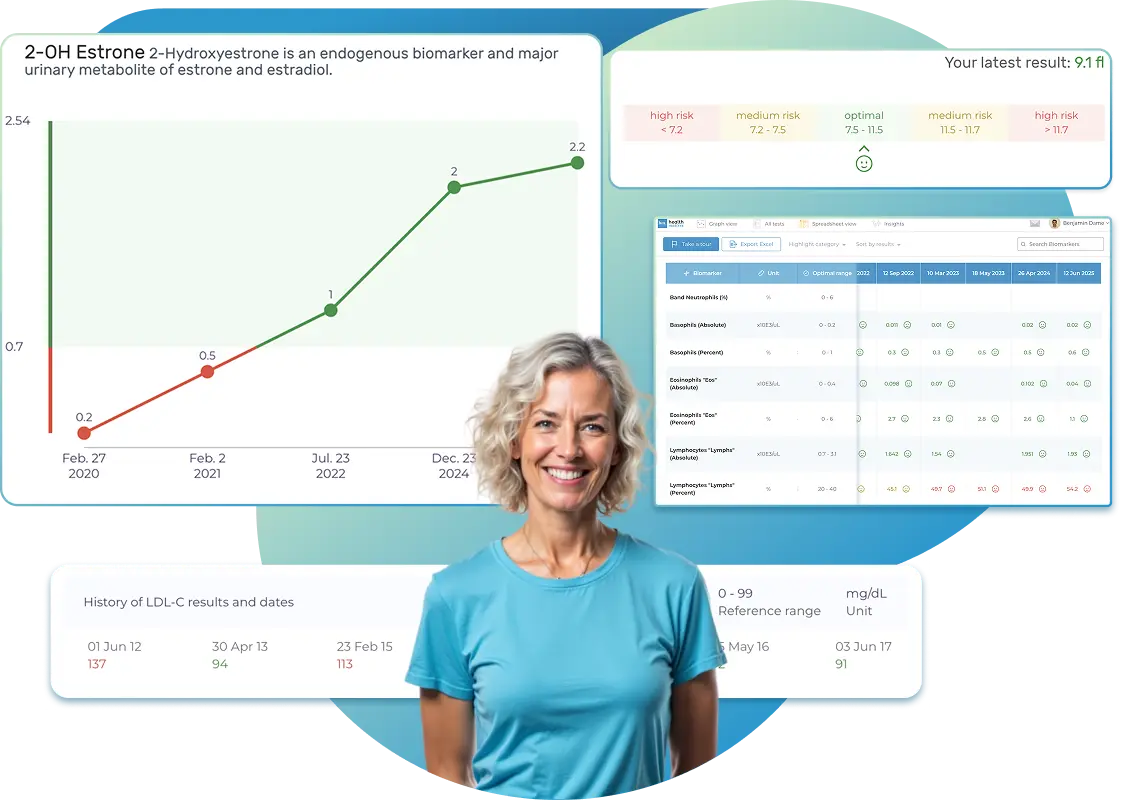
All Your Lab Results.
One Simple Dashboard.
Import, Track, and Share Your Lab Results Easily
Import, Track, and Share Your Lab Results
Import lab results from multiple providers, track changes over time, customize your reference ranges, and get clear explanations for each result. Everything is stored securely, exportable in one organized file, and shareable with your doctor—or anyone you choose.
Cancel or upgrade anytime
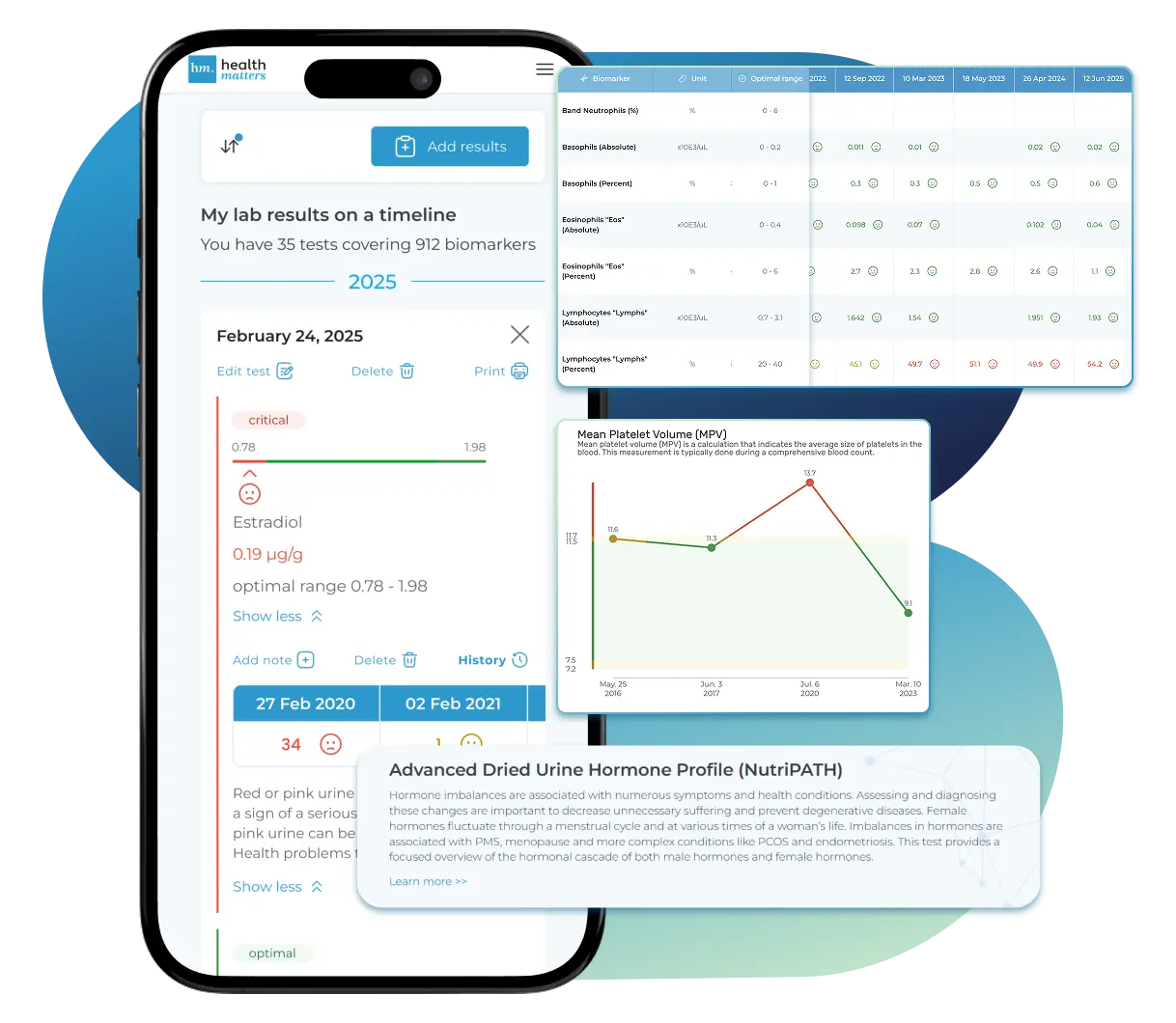
Laboratories
Bring All Your Lab Results Together — In One Place
We accept reports from any lab, so you can easily collect and organize all your health information in one secure spot.
Pricing Table
Gather Your Lab History — and Finally Make Sense of It
Finally, Your Lab Results Organized and Clear
Personal plans
$79/ year
Advanced Plan
Access your lab reports, explanations, and tracking tools.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 5 reports entered for you
- Cancel or upgrade anytime
$250/ once
Unlimited Account
Pay once, access everything—no monthly fees, no limits.
- Import lab results from any provider
- Track all results with visual tools
- Customize your reference ranges
- Export your full lab history anytime
- Share results securely with anyone
- Receive 10 reports entered for you
- No subscriptions. No extra fees.
$45/ month
Pro Monthly
Designed for professionals managing their clients' lab reports
- Import lab results from any provider
- Track lab results for multiple clients
- Customize reference ranges per client
- Export lab histories and reports
- Begin with first report entered by us
- Cancel or upgrade anytime
About membership
What's included in a Healthmatters membership
 Import Lab Results from Any Source
Import Lab Results from Any Source
 See Your Health Timeline
See Your Health Timeline
 Understand What Your Results Mean
Understand What Your Results Mean
 Visualize Your Results
Visualize Your Results
 Data Entry Service for Your Reports
Data Entry Service for Your Reports
 Securely Share With Anyone You Trust
Securely Share With Anyone You Trust
Let Your Lab Results Tell the Full Story
Once your results are in one place, see the bigger picture — track trends over time, compare data side by side, export your full history, and share securely with anyone you trust.





Bring all your results together to compare, track progress, export your history, and share securely.
What Healthmatters Members Are Saying
Frequently asked questions
Healthmatters is a personal health dashboard that helps you organize and understand your lab results. It collects and displays your medical test data from any lab in one secure, easy-to-use platform.
- Individuals who want to track and understand their health over time.
- Health professionals, such as doctors, nutritionists, and wellness coaches, need to manage and interpret lab data for their clients.
With a Healthmatters account, you can:
- Upload lab reports from any lab
- View your data in interactive graphs, tables, and timelines
- Track trends and monitor changes over time
- Customize your reference ranges
- Export and share your full lab history
- Access your results anytime, from any device
Professionals can also analyze client data more efficiently and save time managing lab reports.
Healthmatters.io personal account provides in-depth research on 10000+ biomarkers, including information and suggestions for test panels such as, but not limited to:
- The GI Effects® Comprehensive Stool Profile,
- GI-MAP,
- The NutrEval FMV®,
- The ION Profile,
- Amino Acids Profile,
- Dried Urine Test for Comprehensive Hormones (DUTCH),
- Organic Acids Test,
- Organix Comprehensive Profile,
- Toxic Metals,
- Complete Blood Count (CBC),
- Metabolic panel,
- Thyroid panel,
- Lipid Panel,
- Urinalysis,
- And many, many more.
You can combine all test reports inside your Healthmatters account and keep them in one place. It gives you an excellent overview of all your health data. Once you retest, you can add new results and compare them.
If you are still determining whether Healthmatters support your lab results, the rule is that if you can test it, you can upload it to Healthmatters.
We implement proven measures to keep your data safe.
At HealthMatters, we're committed to maintaining the security and confidentiality of your personal information. We've put industry-leading security standards in place to help protect against the loss, misuse, or alteration of the information under our control. We use procedural, physical, and electronic security methods designed to prevent unauthorized people from getting access to this information. Our internal code of conduct adds additional privacy protection. All data is backed up multiple times a day and encrypted using SSL certificates. See our Privacy Policy for more details.
















